Abstract
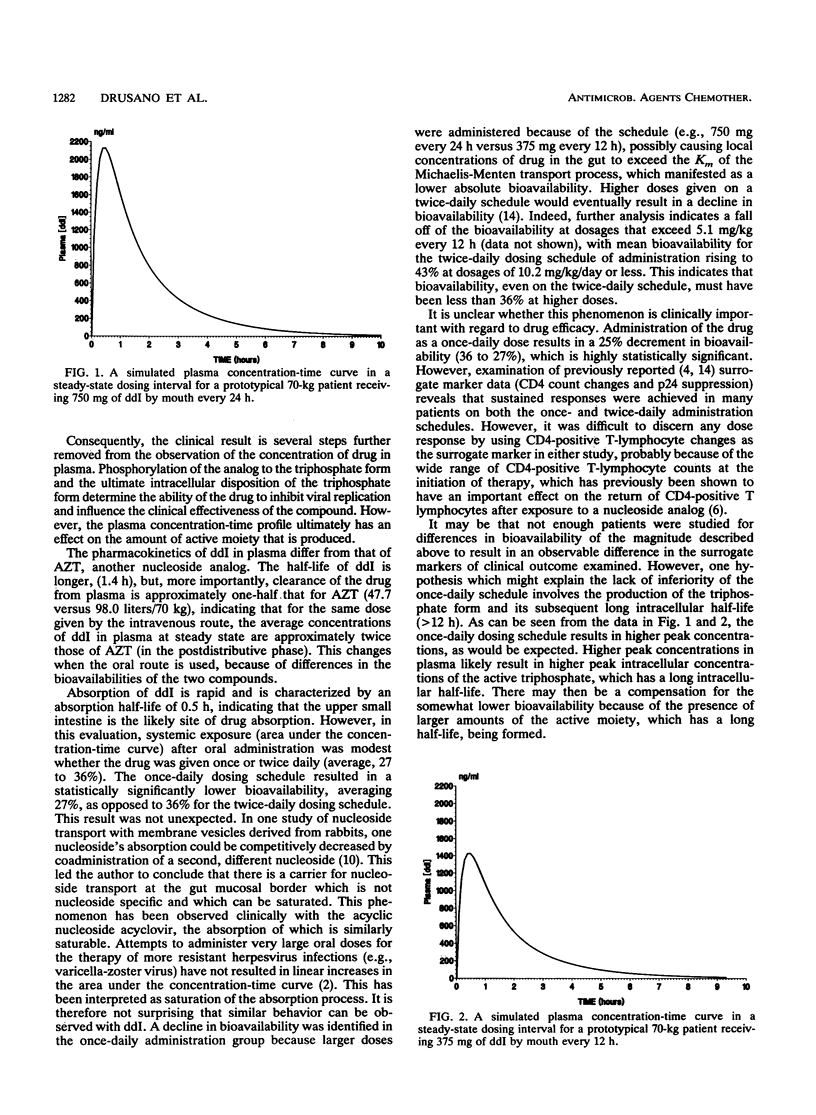
The objective of this study was to determine the population pharmacokinetic parameters and the extent of absorption of 2',3'-dideoxyinosine, a nucleoside analog with activity against human immunodeficiency virus in vitro and in vivo, after oral and intravenous administration through the use of NON-linear Mixed Effects Modeling. The data were drawn from the pharmacokinetics section of an open-label, multicenter phase I study. One center administered ddI on a once-daily schedule. The other centers administered the drug once every 12 h. Drug was administered intravenously, and the plasma concentration-time profile was determined. Patients were then given the drug orally at twice the dose used in the intravenous portion of the study, and the pharmacokinetic profile was again determined. A 40-fold range of doses was examined. Forty-six human immunodeficiency virus-infected patients were studied. Concentrations in plasma were determined by high-pressure liquid chromatography. Clearance of the drug from plasma was 47.7 liters/h/70 kg of body weight. The terminal half-life was 1.4 h. The volume of distribution in the central compartment was 18.8 liters/70 kg. Absorption was rapid, with an absorption half-life of 0.52 h. Bioavailability with once-daily administration was 27%. For twice-daily administration, bioavailability rose to 36%. This difference was significant (P much less than 0.01). For doses of less than or equal to 5.1 mg/kg given every 12 h (10.2 mg/kg/day), bioavailability was 41%. We conclude that once-daily administration results in lower mean bioavailability, probably because of a saturation of the absorption process similar to that seen with acyclovir. This difference in bioavailability on the basis of the administration schedule explains the different short-term maximal tolerated doses identified in phase I trials of this agent.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brigden D., Whiteman P. The clinical pharmacology of acyclovir and its prodrugs. Scand J Infect Dis Suppl. 1985;47:33–39. [PubMed] [Google Scholar]
- Cooley T. P., Kunches L. M., Saunders C. A., Perkins C. J., Kelley S. L., McLaren C., McCaffrey R. P., Liebman H. A. Treatment of AIDS and AIDS-related complex with 2',3'-dideoxyinosine given once daily. Rev Infect Dis. 1990 Jul-Aug;12 (Suppl 5):S552–S560. doi: 10.1093/clinids/12.supplement_5.s552. [DOI] [PubMed] [Google Scholar]
- Cooley T. P., Kunches L. M., Saunders C. A., Ritter J. K., Perkins C. J., McLaren C., McCaffrey R. P., Liebman H. A. Once-daily administration of 2',3'-dideoxyinosine (ddI) in patients with the acquired immunodeficiency syndrome or AIDS-related complex. Results of a Phase I trial. N Engl J Med. 1990 May 10;322(19):1340–1345. doi: 10.1056/NEJM199005103221902. [DOI] [PubMed] [Google Scholar]
- Drusano G. L. Role of pharmacokinetics in the outcome of infections. Antimicrob Agents Chemother. 1988 Mar;32(3):289–297. doi: 10.1128/aac.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drusano G. L., Yuen G. J., Lambert J. S., Seidlin M., Dolin R., Valentine F. T. Relationship between dideoxyinosine exposure, CD4 counts, and p24 antigen levels in human immunodeficiency virus infection. A phase I trial. Ann Intern Med. 1992 Apr 1;116(7):562–566. doi: 10.7326/0003-4819-116-7-562. [DOI] [PubMed] [Google Scholar]
- Fischl M. A., Richman D. D., Grieco M. H., Gottlieb M. S., Volberding P. A., Laskin O. L., Leedom J. M., Groopman J. E., Mildvan D., Schooley R. T. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- Frick L. W., Nelson D. J., St Clair M. H., Furman P. A., Krenitsky T. A. Effects of 3'-azido-3'-deoxythymidine on the deoxynucleotide triphosphate pools of cultured human cells. Biochem Biophys Res Commun. 1988 Jul 15;154(1):124–129. doi: 10.1016/0006-291x(88)90659-6. [DOI] [PubMed] [Google Scholar]
- Ho H. T., Hitchcock M. J. Cellular pharmacology of 2',3'-dideoxy-2',3'-didehydrothymidine, a nucleoside analog active against human immunodeficiency virus. Antimicrob Agents Chemother. 1989 Jun;33(6):844–849. doi: 10.1128/aac.33.6.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis S. M. Characterization of sodium-dependent nucleoside transport in rabbit intestinal brush-border membrane vesicles. Biochim Biophys Acta. 1989 Feb 13;979(1):132–138. doi: 10.1016/0005-2736(89)90533-6. [DOI] [PubMed] [Google Scholar]
- Klecker R. W., Jr, Collins J. M., Yarchoan R., Thomas R., Jenkins J. F., Broder S., Myers C. E. Plasma and cerebrospinal fluid pharmacokinetics of 3'-azido-3'-deoxythymidine: a novel pyrimidine analog with potential application for the treatment of patients with AIDS and related diseases. Clin Pharmacol Ther. 1987 Apr;41(4):407–412. doi: 10.1038/clpt.1987.49. [DOI] [PubMed] [Google Scholar]
- Knupp C. A., Shyu W. C., Dolin R., Valentine F. T., McLaren C., Martin R. R., Pittman K. A., Barbhaiya R. H. Pharmacokinetics of didanosine in patients with acquired immunodeficiency syndrome or acquired immunodeficiency syndrome-related complex. Clin Pharmacol Ther. 1991 May;49(5):523–535. doi: 10.1038/clpt.1991.63. [DOI] [PubMed] [Google Scholar]
- Knupp C. A., Stancato F. A., Papp E. A., Barbhaiya R. H. Quantitation of didanosine in human plasma and urine by high-performance liquid chromatography. J Chromatogr. 1990 Nov 30;533:282–290. doi: 10.1016/s0378-4347(00)82215-x. [DOI] [PubMed] [Google Scholar]
- Lambert J. S., Seidlin M., Reichman R. C., Plank C. S., Laverty M., Morse G. D., Knupp C., McLaren C., Pettinelli C., Valentine F. T. 2',3'-dideoxyinosine (ddI) in patients with the acquired immunodeficiency syndrome or AIDS-related complex. A phase I trial. N Engl J Med. 1990 May 10;322(19):1333–1340. doi: 10.1056/NEJM199005103221901. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Darby G., Richman D. D. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science. 1989 Mar 31;243(4899):1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- Moore R. D., Lietman P. S., Smith C. R. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987 Jan;155(1):93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- Pizzo P. A., Eddy J., Falloon J., Balis F. M., Murphy R. F., Moss H., Wolters P., Brouwers P., Jarosinski P., Rubin M. Effect of continuous intravenous infusion of zidovudine (AZT) in children with symptomatic HIV infection. N Engl J Med. 1988 Oct 6;319(14):889–896. doi: 10.1056/NEJM198810063191401. [DOI] [PubMed] [Google Scholar]
- Schentag J. J., Smith I. L., Swanson D. J., DeAngelis C., Fracasso J. E., Vari A., Vance J. W. Role for dual individualization with cefmenoxime. Am J Med. 1984 Dec 21;77(6A):43–50. doi: 10.1016/s0002-9343(84)80074-1. [DOI] [PubMed] [Google Scholar]
- Sheiner L. B., Rosenberg B., Marathe V. V. Estimation of population characteristics of pharmacokinetic parameters from routine clinical data. J Pharmacokinet Biopharm. 1977 Oct;5(5):445–479. doi: 10.1007/BF01061728. [DOI] [PubMed] [Google Scholar]
- Volberding P. A., Lagakos S. W., Koch M. A., Pettinelli C., Myers M. W., Booth D. K., Balfour H. H., Jr, Reichman R. C., Bartlett J. A., Hirsch M. S. Zidovudine in asymptomatic human immunodeficiency virus infection. A controlled trial in persons with fewer than 500 CD4-positive cells per cubic millimeter. The AIDS Clinical Trials Group of the National Institute of Allergy and Infectious Diseases. N Engl J Med. 1990 Apr 5;322(14):941–949. doi: 10.1056/NEJM199004053221401. [DOI] [PubMed] [Google Scholar]
- Yarchoan R., Mitsuya H., Thomas R. V., Pluda J. M., Hartman N. R., Perno C. F., Marczyk K. S., Allain J. P., Johns D. G., Broder S. In vivo activity against HIV and favorable toxicity profile of 2',3'-dideoxyinosine. Science. 1989 Jul 28;245(4916):412–415. doi: 10.1126/science.2502840. [DOI] [PubMed] [Google Scholar]



