Abstract
In pulmonary arterial hypertension (PAH), antiapoptotic, proliferative, and inflammatory diatheses converge to create an obstructive vasculopathy. A selective down-regulation of the Kv channel Kv1.5 has been described in human and animal PAH. The resultant increase in intracellular free Ca2+ ([Ca2+]i) and K+ ([K+]i) concentrations explains the pulmonary artery smooth muscle cell (PASMC) contraction, proliferation and resistance to apoptosis. The recently described PASMC hyperpolarized mitochondria and increased bcl-2 levels also contribute to apoptosis resistance in PAH. The cause of the Kv1.5, mitochondrial, and inflammatory abnormalities remains unknown. We hypothesized that these abnormalities can be explained in part by an activation of NFAT (nuclear factor of activated T cells), a Ca2+/calcineurin-sensitive transcription factor. We studied PASMC and lungs from six patients with and four without PAH and blood from 23 PAH patients and 10 healthy volunteers. Compared with normal, PAH PASMC had decreased Kv current and Kv1.5 expression and increased [Ca2+]i, [K+]i, mitochondrial potential (ΔΨm), and bcl-2 levels. PAH but not normal PASMC and lungs showed activation of NFATc2. Inhibition of NFATc2 by VIVIT or cyclosporine restored Kv1.5 expression and current, decreased [Ca2+]i, [K+]i, bcl-2, and ΔΨm, leading to decreased proliferation and increased apoptosis in vitro. In vivo, cyclosporine decreased established rat monocrotaline-PAH. NFATc2 levels were increased in circulating leukocytes in PAH versus healthy volunteers. CD3-positive lymphocytes with activated NFATc2 were seen in the arterial wall in PAH but not normal lungs. The generalized activation of NFAT in human and experimental PAH might regulate the ionic, mitochondrial, and inflammatory remodeling and be a therapeutic target and biomarker.
Keywords: apoptosis, K+ channel, mitochondria
The remodeled pulmonary arteries (PA) in pulmonary arterial hypertension (PAH) are characterized by vasoconstriction, suppressed apoptosis, and increased proliferation within the vascular wall (1). Endothelial dysfunction, an early abnormality in PAH, leads to an increase in vasoconstrictors (like endothelin) over vasodilators (like prostacyclin) (2). Correction of these abnormalities by blockade of the endothelin axis or enhancement of the prostacyclin axis is the basis of the current treatment for PAH; however the morbidity and mortality remains high (3). More effective therapies for PAH will have to directly and selectively target the mechanisms leading to the proproliferative and antiapoptotic environment in the vascular wall; the recently described abnormalities in the PA smooth muscle cell (PASMC) K+ channels and mitochondria are such mechanisms (2, 4–7).
A selective down-regulation of PASMC Kv channels, like Kv1.5, has been described both in human (7) and animal models of PAH (6, 8–10). This causes depolarization, opening of the voltage-gated Ca2+ channels, and increase in intracellular free Ca2+ concentration ([Ca2+]i). The increased [Ca2+]i can explain both the vasoconstriction and the PASMC proliferation (11). Kv1.5 channel down-regulation also causes an increase in intracellular K+ concentration ([K+]i) (inhibiting the efflux of K+ down its concentration gradient), which results in caspase inhibition. In several cell types, ranging from neurons, cancer cells, and PASMC, increased [K+]i directly inhibits caspases and apoptosis (12–14). The mechanism for the down-regulation of Kv1.5 channel expression in PAH remains unknown.
In human and animal PAH, there appears to be a mitochondrial remodeling, as suggested by hyperpolarized PASMC mitochondria, compared with normal PASMC (4, 5). The increased mitochondrial membrane potential (ΔΨm) might be a marker of apoptosis resistance, because apoptosis is typically initiated by decrease in ΔΨm and efflux of proapoptotic mediators. Most carcinomas have increased ΔΨm (15, 16). ΔΨm is regulated by metabolic or molecular mechanisms. Members of the bcl-2 family can directly control ΔΨm and apoptosis by interacting with the mitochondrial voltage-dependent anion channel or forming channels in mitochondrial membranes. The antiapoptotic bcl-2 prevents the decrease in ΔΨm induced by proapoptotic stimuli by enhancing H+ efflux from the mitochondria (17) and is up-regulated in human PAH (18).
These PAH abnormalities (down-regulated Kv channels, up-regulated bcl-2, hyperpolarized mitochondria) at first appear unrelated. However, there is evidence that they might be related. For example, bcl-2 inhibits Kv channels, whereas the proapoptotic cytochrome c activates Kv channels in PASMC (14). In addition, dichloroacetate, an inhibitor of the mitochondrial enzyme pyruvate dehydrogenase kinase, reverses the mitochondrial hyperpolarization in both PAH (6) and cancer (12) and inhibits vascular remodeling and cancer growth; remarkably, in both conditions, it also reverses the down-regulation of Kv1.5, suggesting the presence of a mitochondria-Kv channel axis. We hypothesized that the Kv channel and mitochondria abnormalities might be “choreographed” by a “master regulator,” such as a transcription factor (19).
Recent observations alerted us to the possibility that the nuclear factor of activated T cells (NFAT) might be involved in PAH. First, NFAT activation causes myocardial down-regulation of Kv1.5 (20). Second, endothelin [which is up-regulated in PAH (2)] activates NFAT, which in turn increases bcl-2 expression, contributing to the prosurvival and antiapoptotic effects of endothelin in the heart (21). Third, NFAT regulates the transcription of several metabolic and mitocondrial genes [like adenylosuccinate synthetase 1 (22), heart fatty acid binding protein, pyruvate decarboxylase, cytochrome c oxidase, and succinate dehydrogenase (23)]. In NFAT knockout models, embryonic lethality follows myocardial mitochondrial dysfunction due to suppressed respiration and oxidative capacity (23), all of which are reversed by myocardial NFAT overexpression (23).
NFAT, originally described in T cells, is a master activator of T cells, increasing the transcription of multiple inflammatory mediators and activating T and B cells (24). Increased [Ca2+]i activates calcineurin, which dephosphorylates cytoplasmic NFAT, allowing its entry to the nucleus, where it forms complexes with other transcription factors (like GATA or AP1) and regulates gene transcription (24). The role of NFAT is now recognized in many cellular functions, beyond inflammation (19, 24, 25). Inflammation also plays an understudied role in PAH; increased inflammatory mediators have been reported in serum and within the remodeled PA of PAH patients and a clinical association exists between PAH and autoimmune diseases like scleroderma (26–28). We hypothesized that the ionic, mitochondrial, and inflammatory remodeling in PAH have a common denominator, i.e., a “generalized” activation of NFAT. This “unifying” hypothesis for the pathogenesis of PAH also suggests that NFAT inhibition might be therapeutically beneficial.
Results
NFATc2 Activation in Human PAH, But Not Normal PAs, in Vivo.
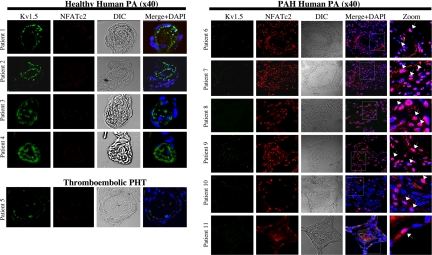
We studied 11 human lungs, 6 with PAH, 4 normals (transplant donors), and 1 with thromboembolic disease [supporting information (SI) Table 1]. In normal and secondary pulmonary hypertension small PAs, there was low expression of NFATc2, which was almost always cytoplasmic, i.e., inactive (Fig. 1). In the same small PAs, there was strong expression of Kv1.5. In contrast, in all PAH PAs, there was up-regulation of NFATc2, which was translocated to the nucleus (i.e., activated) in most cells within the PA wall. In these remodeled PAs, there was a significant down-regulation of Kv1.5. Staining with only secondary antibodies revealed no signal, supporting the antibody specificity (SI Fig. 7A). To study whether NFAT activation is sustained in vitro and whether NFAT dynamically regulates Kv channel expression, mitochondrial function, and apoptosis, we used human cultured normal and PAH PASMC.
Fig. 1.
NFATc2 and Kv1.5 in human normal and PAH pulmonary arteries (PA). Confocal immunohistochemistry of human PAs from both normal and PAH patients (see also SI Fig. 11). PAH patients show up-regulated and activated NFATc2 (colocalized with the nucleus, which is stained with DAPI) within the PA wall. The anatomy of the vessels (lumen, media) can be seen in the dissemintated intravascular coagulation windows. Examples of nuclear NFATc2 are shown by arrows in the merged “zoom” column. In the same PAs, Kv1.5 expression is significantly down-regulated. In contrast, in four patients with normal pulmonary circulation and one patient with thromboembolic pulmonary hypertension, Kv1.5 expression is strong and NFAT2c expression is low and not nuclear.
NFATc2 Activation in iPAH PASMC.
Compared with normal, idiopathic PAH (iPAH) PASMC have less total and 4-aminopyridine-sensitive K+ current density (i.e., Kv) and depolarized membrane potential (Em) (Fig. 2A). This is associated with increased [Ca2+]i (Fig. 2B), activated NFATc2 (Fig. 2C), and decreased Kv1.5 expression (Fig. 2D). Whereas >60% of iPAH PASMC show NFATc2 activation (Fig. 2C), only ≈20% are positive for activated NFATc3 (SI Fig. 7B). NFATc4 is present in several iPAH PASMC, but is activated in <5% of cells (SI Fig. 7B). None of the NFAT isoforms are significantly present or active in normal PASMC. Thus, NFATc2 is the predominant NFAT isoform in iPAH PASMC.
Fig. 2.
NFATc2 and Kv1.5 in PAH PASMC. (A) Compared with normal PASMCs, iPAH PASMCs have significantly decreased whole-cell K+ current density and 4-AP-sensitive K+ current, membrane potential (Em), cell capacitance, and [K+]i. All of these are normalized by VIVIT or cyclosporine (CSA) (n = 11–12 per group). (B) [Ca2+]i measured with fluo-3 acetoxymethyl ester is significantly increased in iPAH compared with normal PASMC and is normalized by VIVIT (n = 30–35 per group). ∗, P < 0.05 vs. iPAH. (C) Immunocytochemistry (n ≈ 70 cells per group per experiment, three experiments) for NFATc2 (green) and the nucleus (blue) reveals that compared with normal cells, the majority of iPAH PASMC have activated NFATc2 (green within the nucleus). VIVIT or CSA, but not antennapedia, reverse the NFATc2 activation. (D) iPAH PASMC have decreased levels of Kv1.5 protein, compared with healthy PASMC, which is reversed by 48 h of exposure to VIVIT or CSA. Immunoblots in cultured PASMC were performed in triplicate; representative and mean densitometry data are shown. ∗, P < 0.05 vs. healthy controls.
We then used a direct and specific inhibitor of NFAT [i.e., VIVIT (29)] and an indirect inhibitor [i.e., cyclosporine A (CSA)] to dynamically study NFAT activation and its downstream effects. We used a commercially available form of VIVIT with enhanced cell permeability due to the addition of an 11-arginine repeat. To address potential nonspecific effects of this small peptide, we also studied a nonactive cell-permeable small peptide with a similar structure (high arginine and lysine content) used to facilitate the cellular entry of viruses or drugs [i.e., antennapedia (30)]. Both VIVIT (but not antennapedia) and CSA-treated iPAH PASMC have cytoplasmic and not nuclear NFATc2, increased outward K+ current (and decreased [K+]); increased Em, and decreased [Ca2+]i (Fig. 2), with values essentially restored to those of normal PASMC. Most of the VIVIT-induced current is 4-AP sensitive, in keeping with Kv1.5 up-regulation (Fig. 2 A and D). Both VIVIT and CSA-treated PASMCs are also smaller, as shown by the decreased capacitance, an electrophysiological surrogate for cell size (Fig. 2A). This is likely due to both efflux of K+ and net osmotic loss and it is compatible with early apoptosis, which is associated with cell shrinkage (14).
We have shown that PAH PASMC have hyperpolarized mitochondria compared with healthy PASMC and hypothesized that this is a marker of apoptosis resistance (4–6). Both VIVIT and CSA (but not antennapedia) depolarized PASMC mitochondria, essentially normalizing ΔΨm (Fig. 3A). The mitochondrial depolarization led to efflux of the proapoptotic cytochrome c (Fig. 3B) and induction of iPAH PASMC apoptosis (Fig. 3C). The induction of apoptosis, and not necrosis, was shown because the treated PASMC were annexin-positive but propidium iodide-negative. Both CSA and VIVIT (but not antennapedia) increased the percentage of TUNEL-positive cells and decreased PASMC proliferation (percentage of proliferating cell nuclear antigen-positive cells). This decrease in proliferation is related to the Ca2+-lowering effects of VIVIT and CSA, which is in turn related to their ability to reverse the Kv channel down-regulation.
Fig. 3.
NFATc2 regulates mitochondrial function, Bcl-2, and apoptosis in PAH PASMC. (A) iPAH PASMC mitochondria are hyperpolarized compared with normal and normalized by VIVIT or CSA (n≈ 50 per group per experiment, three experiments). (B) Although in untreated iPAH and healthy cells cytochrome c (green) colocalizes with the mitochondria (stained by mitotracker red), in the VIVIT-treated cells, cytochrome c is cytoplasmic (diffuse staining pattern). (C) VIVIT and CSA, but not antennapedia, induce apoptosis (% TUNEL-positive cells; annexin but not propidium iodide-positive cells) and decrease proliferation (% PCNA-positive cells) in iPAH PASMCs. (n ≈ 70 per group per experiment, three experiments). (D) (Left) Bcl-2 expression is increased in iPAH compared with healthy cells, which is reversed by VIVIT and CSA. (Right) Although in iPAH bcl-2 colocalizes with mitotracker (yellow staining), after VIVIT treatment, bcl-2 dissociates from mitochondria. ∗, P < 0.05 vs. iPAH.
iPAH PASMC have increased expression of the antiapoptotic bcl-2 compared with normal PASMC, and this is reversed by VIVIT (Fig. 3D). Moreover, VIVIT results in displacement of bcl-2 from the mitochondria; although in PAH PASMC bcl-2 colocalizes with the mitochondrial marker mitotracker, in VIVIT-treated cells this colocalization is lost (Fig. 3D). To further study the dynamic nature of the effects of NFAT, we studied normal PASMC exposed to chronic hypoxia, which causes a phenotype similar to iPAH, characterized by down-regulation of Kv channels, increased [Ca2+]i, and proliferation and hyperpolarization of mitochondria (4, 9, 31).
Dynamic Regulation of Kv1.5, Mitochondria and Proliferation/Apoptosis by NFATc2 in Normal PASMC.
Exposure of normal human PASMC to hypoxia for 96 h caused the expected decrease in Ik, evident in negative potentials close to the resting Em (SI Fig. 8A), and increased [Ca2+]i (SI Fig 8B). Like iPAH PASMC, hypoxic PASMC had activated NFATc2 and decreased Kv1.5 expression (SI Figs. 8C and 9A). Despite ongoing hypoxia, VIVIT inhibited the nuclear translocation of NFATc2 and reversed the down-regulation of Kv1.5, increasing Ik, repolarizing Em, and normalizing [Ca2+]i (Figs. 7 A–C and 9A). VIVIT did not alter the expression of K+ channels from different families, like the large conductance calcium-activated K+ channels (BKCa) (SI Fig. 9A). The hypoxia-induced increase in PASMC ΔΨm (4, 5) was reversed by VIVIT (SI Fig. 8D). The VIVIT-induced mitochondrial depolarization led to apoptosis, shown by efflux of cytochrome c (SI Fig. 9B), increase in %TUNEL-positive cells (SI Figs. 8D and 9C), activation of caspase 9 (SI Fig. 9C), and staining for annexin (SI Fig. 10A). VIVIT also decreased proliferation (decreased BrdU uptake and proliferating cell nuclear antigen expression) (SI Figs. 8D and 10B).
NFATc2 and Inflammation in PAH.
Because products of activated immune cells, like interleukins, are increased in PAH patients, we determined whether activated NFAT (in addition to the lungs) can also be detected in the circulating cells. We studied two groups, in addition to control normal volunteers: a group of scleroderma-PAH (scl-PAH) patients, which, along with iPAH, belongs to the “PAH superfamily” (3) (SI Table 1). scl-PAH is characterized by a strong inflammatory component and worse prognosis than iPAH (3). We also studied a group with severe secondary pulmonary hypertension (thromboembolic disease, pulmonary fibrosis) to exclude the possibility that NFAT activation in circulating cells might be caused by the stress of cells passing through the remodeled PAs. CD3-positive T lymphocytes were found in the resistance PAs from all PAH patients but not in normal controls (Fig. 4A and SI Table 2). The majority of CD3-positive cells also showed NFATc2 activation.
Fig. 4.
NFAT in circulating blood cells in PAH. (A) Control patients do not show any infiltration with CD-3-positive T cells (red), or NFATc2 expression (green) in PAs; in PAH patients there are cells positive for both CD3 and activated NFATc2 attached to the endothelium (Upper) and within the PA wall (Lower). (B) NFATc2 is active in most circulating white cells in PAH but in none of the healthy patients (representative immunocytochemistry is shown). Buffy coat NFATc2 mRNA levels are higher in PAH patients (particularly scleroderma-PAH) compared with healthy controls or secondary pulmonary hypertension despite similar levels of pulmonary vascular resistance (PVR) with the latter (SI Table 2). However, ther was no correlation between NFATc2 levels and pulmonary vascular resistance in these cohorts. ∗, P < 0.05 vs. healthy controls.
We then studied white blood cells from the buffy coat of fresh blood. In 9 of 11 iPAH patients and in all scl-PAH, there was evidence of nuclear NFATc2. In contrast, in healthy volunteers (who did not have any evidence of cardiovascular, systemic disease, active or recent inflammation, or infection), cells had either no detectable or inactive (cytoplasmic) NFATc2 (Fig. 4B). PAH patients also had higher levels of NFATc2 mRNA (qRT-PCR in buffy coat samples), compared with normal volunteers or secondary pulmonary hypertension. Although 2 of 11 iPAH patients had NFATc2 levels that overlapped those in normal or secondary pulmonary hypertension subjects, every scl-PAH patient had much higher levels of NFATc2 in circulating white cells (Fig. 4B).
NFAT Inhibition Reverses Established Rat PAH.
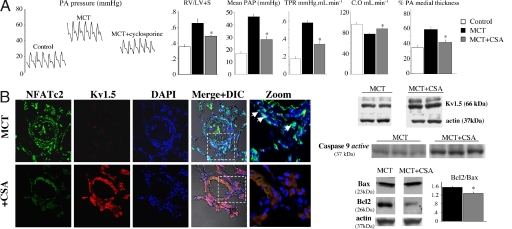
The reversal of iPAH phenotype in isolated human PASMC suggested that CSA might reverse PAH in vivo. After 2 weeks of oral CSA therapy (1 mg/kg, similar to the dose used clinically) in rats with established PAH [3 weeks post injection of monocrotaline (4–6)], total pulmonary vascular resistance and mean PA pressure were decreased and cardiac output increased (Fig. 5A). CSA therapy decreased right ventricular hypertrophy (Fig. 5A) and medial hypertrophy of small–medium PAs (≈100–400 μm) (Fig. 5B). CSA up-regulated PA Kv1.5 in vivo, shown by immunohistochemistry and immunoblots in isolated PAs (Fig. 5B). In the same PAs, net NFATc2 expression was decreased and expressed NFAT was inactive (not in nucleus). PAs from CSA-treated rats had increased levels of activated caspase-9 and decreased ratio of bcl-2/bax, indicative of enhanced mitochondria-dependent apoptosis (Fig. 5B).
Fig. 5.
Cyclosporine A reverses PAH. (A) Three weeks after MCT injection, rats were treated with CSA (1 mg/kg per os) for 2 weeks. CSA decreased mean PA pressure, total pulmonary vascular resistance, and right ventricular hypertrophy; increased cardiac output; and improved vascular remodeling (% medial thickness) (n = 5–6 per group). (B) Both immunohistochemistry and immunoblots (resistance PAs from four rats pooled per lane) show that CSA decreases NFATc2 activation and expression and restores Kv1.5 expression, mimicking VIVIT. PASMCs with activated NFATc2 are shown by arrows in the merged/zoom panels. CSA increases activated caspase 9 and decreases the bcl-2/bax ratio (a representative immunoblot from pooled PAs and densitometry data from three experiments are shown). ∗, P < 0.05 vs. MCT-PAH.
Discussion
We show that NFAT is activated in human and animal PAH and that this activation explains several molecular hallmarks of this mysterious disease. NFAT activation contributes significantly to Kv1.5 down-regulation, bcl-2 up-regulation, and mitochondrial hyperpolarization, all of which contribute to a resistance to apoptosis within the PA wall. We provide evidence of a generalized activation of NFAT in circulating cells that could contribute to the inflammatory component of vascular remodeling and form the basis of “biomarker” discovery strategies. We demonstrate that NFAT inhibition is therapeutically beneficial in an accepted experimental model of PAH. Recently, there is interest in developing specific NFAT inhibitors, such as the VIVIT peptide we used, which does not inhibit calcineurin activity nonspecifically, but blocks the docking of calcineurin on NFAT itself (29) for the treatment of cardiac hypertrophy and failure (32). Such “transcriptional factor”-targeted therapies may bypass the nonspecific effects of other clinically used NFAT inhibitors like CSA and FK506 and therefore avoid many of their toxicities (32). Although the clinical use of peptides is challenging, limited by antigenicity, proteolysis, or selective tissue delivery, our work suggests that novel NFAT-inhibiting strategies should be considered in human PAH. An advantage of treating PAH with NFAT inhibitors is that they might not only reverse the PA remodeling through effects on PASMC and inflammatory cells but might simultaneously contribute to reversing RV hypertrophy through primary effects on cardiomyocytes. RV hypertrophy/failure is the most important predictor of morbidity and mortality in PAH (33). This combination of parallel beneficial effects in both the vascular bed and the heart is highly desirable and might be the first example of a comprehensive, “transcriptional” therapy in the diseased RV-pulmonary circulation “unit” in PAH.
Kv Channels and PAH.
The first description of selective down-regulation of Kv channels in PAH, particularly Kv1.5, suggested that these channels are dysfunctional (7), perhaps with a genetic basis. Our work suggests that this is not the case; rather, Kv channel expression is actively suppressed by activated NFAT. Inhibiting NFAT rapidly restores expression of functional Kv channels in PASMC, normalizing Em and [Ca2+]i in both human iPAH (Fig. 2) and the physiologic ionic remodeling induced by hypoxia (SI Fig. 8).
The PASMC depolarization that follows Kv1.5 inhibition leads to two detrimental ionic consequences: influx of Ca2+ [promoting contraction and proliferation (11)] and accumulation of intracellular K+ [which suppresses apoptosis (14)]. Our contention that Kv1.5 is important in PAH is supported by its down-regulation in human and all available models of PAH, including hypoxia-induced, monocrotaline-induced, the fawn-hooded rat, mice overexpressing the serotonin transporter, or transgenic mice with a conditional mutation in BMPR-II (4–6, 8, 9, 34). Moreover, seemingly unrelated experimental therapies that reverse animal PAH in vivo, including the metabolic modulator Dichloroacetate and inhibition of survivin or the serotonin axis, all result in increased PASMC Kv1.5 expression/activity (5, 6, 8). The regulation of Kv1.5 in all these unrelated models and therapies might have a common denominator, i.e., NFAT. Despite differences in the “proximal” part of these mechanisms they might all share a common “distal” effector in inducing PAH; this is actually a role that has already been proposed for gene transcription by NFAT, i.e., an integrator and “choreographer” of many signaling pathways (19, 24).
Mitochondria and PAH.
We have suggested that a mitochondrial remodeling might contribute to the apoptosis resistance in PAH and cancer (4–6, 12). The role of mitochondria in the pathogenesis of PAH is particularly important because it might help explain why the pathology of PAH is restricted to he pulmonary vessels, sparing the systemic vasculature; given the recently described mitochondrial heterogeneity in the vasculature, where critical differences between PASMC mitochondria and those of systemic arterial smooth muscle cells have been described (35). The described mitochondrial hyperpolarization in iPAH PASMC is compatible with a resistance to apoptosis state but its etiology remains unknown. NFAT inhibition leads to depolarization of the mitochondria, allowing the efflux of proapoptotic mediators in the cytoplasm. This might be explained by its ability to regulate the expression of bcl-2 (Fig. 3D and ref. 21), or several metabolic/mitochondrial enzymes (22, 23). Conversely, it is possible that NFAT activation is secondary to an earlier and more fundamental mitochondrial abnormality. In fact, the “retrograde pathway,” in which NFAT plays an important role, is thought to transmit a “metabolic” signal to the nucleus, to coordinate gene transcription with the appropriate metabolic pathways (36). It is not clear whether a primary signal for the pathogenesis of PAH comes from the mitochondria or from NFAT.
Inflammation and PAH.
The role of inflammation in vascular remodeling and PAH is incompletely understood. We provide preliminary evidence that NFATc2-activated circulating inflammatory cells are found in the blood and also within the PA wall in PAH patients (Fig. 4). Several interleukins and TNF-α are increased in patients with PAH (26–28), and interestingly, many of these cytokines are regulated by NFAT (24). The NFATc2 levels in scl-PAH patients' blood were the highest among all other groups (Fig. 4B); interestingly, scl-PAH has the worst prognosis compared with noninflammatory PAH-associated conditions (3). If our results are confirmed in larger cohorts, then NFAT in the peripheral blood (in the absence of other systemic inflammatory conditions) might be a potential biomarker for PAH.
An NFAT-Based Theory for PAH (Fig. 6).
Fig. 6.
An NFAT-based theory for the pathogenesis of PAH and the multiple sites that NFAT-based therapeutic strategies might affect (see Discussion).
The NFAT activation likely has a multifactorial etiology in PAH; however, NFAT might be a critical integrator of multiple signaling pathways, and its downstream effects might explain several and important features of PAH (Fig. 6). Endothelial dysfunction is one of the earliest abnormalities in PAH, resulting in an imbalance of endothelium-derived vasoactive factors; with increased vasoconstrictors (endothelin, thromboxane, 5-HT; all of which lead to an increase in PASMC [Ca2+]i) and decreased vasodilators (like NO or prostacyclin, which in contrast lead to a decrease in PASMC [Ca2+]i) (2). The promoted high [Ca2+]i-state leads to NFAT activation in PASMC and regulation of multiple genes that might positively reinforce NFAT activation. For example, the down-regulation of Kv1.5 will lead to PASMC depolarization and opening of L-type Ca2+ channels and will sustain the increase in [Ca2+]i and thus NFAT activation. The increase in PASMC [Ca2+]i might also be promoted by other possibly primary PASMC abnormalities, like the dys-regulated serotonin transporter, up-regulation of TRP channels (37), or decreased SERCA activity (38). Activation of NFAT will complete a reinforcing positive-feedback loop with the down-regulation of Kv1.5 and may explain why the phenotype is preserved in PASMC in culture, in the absence of endothelium-derived or circulating factors. Multiple NFAT binding elements (i.e., GGAAA) are present in the promoter regions of both the Kv1.5 (20) and bcl-2 (21) genes. Both of these effects will promote a state of proliferation and suppressed mitochondrial-dependent apoptosis. The involvement of NFAT in the inflammatory changes in PAH, and its well known effects in cardiac hypertrophy, suggest that an NFAT inhibition strategy might target PAH with beneficial effects at multiple levels.
The absence of NFAT activation in the PAs (Fig. 1) and the peripheral blood of patients with secondary pulmonary hypertension (Fig. 4) is intriguing and supports that our findings are not secondary to the vascular remodeling and the increased pressures per se; however, the limited examples of this condition in our study do not allow for generalization of this finding.
NFAT Activation in Both PAH and Cancer.
Intriguing similarities in the apoptosis resistance in PAH and in cancer have started being recognized (28). The oncoprotein survivin is expressed in the PAH PA media and a cancer-based strategy to inhibit endogenous survivin leads to reversal of PAH (5). Monocrotaline-PAH has been shown to be reversed by the anticancer drug Gleevec (39). We recently reported that a similar to PAH Kv1.5 and mitochondrial remodeling (down-regulated Kv1.5 and hyperpolarized mitochondria) is present in several cancer cell lines (12). Cancer growth in vitro and in vivo was inhibited by the mitochondria-targeting drug Dichloroacetate, which reversed both the ionic and mitochondrial remodeling in cancer in a manner identical to that in PAH (6). Intriguingly, it appears that this ionic-mitochondrial remodeling in cancer is also mediated by NFATc2 activation (12). Direct inhibition of NFATc2 in cancer cells by VIVIT reversed the down-regulation of Kv1.5, decreasing proliferation and inducing apoptosis. The current and recent findings suggest that the NFAT–Kv channel–mitochondria axis regulates ionic and metabolic remodeling and apoptosis in diverse conditions ranging from vascular disease and myocardial hypertrophy to cancer.
Methods
Confocal Microscopy.
Imaging was performed in frozen or paraffin-embedded tissues or cultured cells, using a Zeiss (Thornwood, NY) LSM 510 model as described in refs. 5 and 12. For details, sources, and strengths for antibodies, see SI Text.
PASMC in Culture.
PASMC cell lines were established from resistance PAs both from iPAH (patient 7) and patients with normal pulmonary circulation (patients 1, 2) as described in ref. 5. After enzymatic isolation, PASMC were kept at 37°C in DMEM supplemented with 10% FBS and 1% PSF (PO2 ≈120 mm Hg/PCO2 ≈40 mm Hg, pH≈7.2). For hypoxic experiments, healthy PASMC were kept for 96 h in a hypoxic incubator (4% O2/PO2 45 ± 5 mm Hg) and pH (≈7.3 ± 0.1) as described in ref. 4. PASMC were treated with the cell-permeable NFAT inhibitor 11R-VIVIT (EMD Biosciences, Mississaga, ON, Canada) for 48 h (4 μM) (29) or antennapedia, (EMD Biosciences) (48 h, 4 μM) (30) or Cyclosporine A (EMD Biosciences) (0.03 mg·ml−1, 48 h). All drugs were dissolved in PBS, and pH was normalized.
Ca2+ measurements, K+ measurements, and electrophysiology were performed as described in refs. 5, 6, and 12. See also SI Text.
Immunoblotting.
PASMC or PAs were collected, and immunoblotting was performed on pooled samples from four T-25 dishes or four rats (25 μg of protein in pooled samples per lane) and normalized to smooth muscle actin, as described in refs. 5, 6, and 12.
Quantitative RT-PCR.
qRT-PCR was performed in an ABI PRISM 7700 Sequence Detector (Applied Biosystems) and commercially available primers (5, 6, and 12).
In Vivo Rat Studies.
Male Sprague–Dawley rats were injected with monocrotaline as described in refs. 5 and 6. Mean PA pressure, cardiac output, total pulmonary resistance, percentage of medial thickness of small-medium PAs (100–400 μm), and right ventricular hypertrophy were measured as described in refs. 5 and 6. Rats with established monocrotaline-PAH were randomized to receive cyclosporine (1 mg/kg per os × 2 weeks), or vehicle (water).
Statistics.
Values are expressed as the mean ± SEM. Intergroup differences were assessed by Kruskal–Wallis or one-way ANOVA, with post hoc analysis, using Fisher's exact test (∗, P < 0.05).
Supplementary Material
Acknowledgments
This work was supported by grants from the Canadian Institutes for Health Research (CIHR) and the Alberta Heritage Foundation for Medical Research (AHFMR) (E.D.M.) and postdoctoral fellowships from CIHR and AHFMR (S.B.).
Abbreviations
- [Ca2+]i
intracellular free Ca2+ concentration
- CSA
cyclosporine A
- [K+]i
intracellular K+ concentration
- Kv
voltage-gated K+ channel
- NFAT
nuclear factor of activated T cell
- PA
pulmonary artery
- PAH
pulmonary arterial hypertension
- PASMC
pulmonary artery smooth muscle cells
- scl-PAH
scleroderma-pulmonary arterial hypertension
- iPAH
idiopathic PAH.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610467104/DC1.
References
- 1.Michelakis ED. Circ Res. 2006;98:172–175. doi: 10.1161/01.RES.0000204572.65400.a5. [DOI] [PubMed] [Google Scholar]
- 2.Archer S, Rich S. Circulation. 2000;102:2781–2791. doi: 10.1161/01.cir.102.22.2781. [DOI] [PubMed] [Google Scholar]
- 3.Archer SL, Michelakis ED. Curr Opin Cardiol. 2006;21:385–392. doi: 10.1097/01.hco.0000231410.07426.9b. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thebaud B, Bonnet S, Haromy A, Harry G, Moudgil R, McMurtry MS, et al. Circulation. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 5.McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, Bonnet S, Puttagunta L, Michelakis ED. J Clin Invest. 2005;115:1479–1491. doi: 10.1172/JCI23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMurtry MS, Bonnet S, Wu X, Dyck JR, Haromy A, Hashimoto K, Michelakis ED. Circ Res. 2004;95:830–840. doi: 10.1161/01.RES.0000145360.16770.9f. [DOI] [PubMed] [Google Scholar]
- 7.Yuan XJ, Wang J, Juhaszova M, Gaine SP, Rubin LJ. Lancet. 1998;351:726–727. doi: 10.1016/S0140-6736(05)78495-6. [DOI] [PubMed] [Google Scholar]
- 8.Guignabert C, Izikki M, Tu LI, Li Z, Zadigue P, Barlier-Mur AM, Hanoun N, Rodman D, Hamon M, Adnot S, Eddahibi S. Circ Res. 2006;98:1323–1330. doi: 10.1161/01.RES.0000222546.45372.a0. [DOI] [PubMed] [Google Scholar]
- 9.Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R, Archer SL. Circulation. 2002;105:244–250. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- 10.Pozeg ZI, Michelakis ED, McMurtry MS, Thebaud B, Wu XC, Dyck JR, Hashimoto K, Wang S, Moudgil R, Harry G, et al. Circulation. 2003;107:2037–2044. doi: 10.1161/01.CIR.0000062688.76508.B3. [DOI] [PubMed] [Google Scholar]
- 11.Platoshyn O, Golovina VA, Bailey CL, Limsuwan A, Krick S, Juhaszova M, Seiden JE, Rubin LJ, Yuan JX. Am J Physiol. 2000;279:C1540–C1549. doi: 10.1152/ajpcell.2000.279.5.C1540. [DOI] [PubMed] [Google Scholar]
- 12.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, et al. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Pal S, Hartnett KA, Nerbonne JM, Levitan ES, Aizenman E. J Neurosci. 2003;23:4798–4802. doi: 10.1523/JNEUROSCI.23-12-04798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Remillard CV, Yuan JX. Am J Physiol. 2004;286:L49–L67. doi: 10.1152/ajplung.00041.2003. [DOI] [PubMed] [Google Scholar]
- 15.Chen LB. Annu Rev Cell Biol. 1988;4:155–181. doi: 10.1146/annurev.cb.04.110188.001103. [DOI] [PubMed] [Google Scholar]
- 16.Heerdt BG, Houston MA, Augenlicht LH. Cancer Res. 2005;65:9861–9867. doi: 10.1158/0008-5472.CAN-05-2444. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu S, Eguchi Y, Kamiike W, Funahashi Y, Mignon A, Lacronique V, Matsuda H, Tsujimoto Y. Proc Natl Acad Sci USA. 1998;95:1455–1459. doi: 10.1073/pnas.95.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geraci MW, Moore M, Gesell T, Yeager ME, Alger L, Golpon H, Gao B, Loyd JE, Tuder RM, Voelkel NF. Circ Res. 2001;88:555–562. doi: 10.1161/01.res.88.6.555. [DOI] [PubMed] [Google Scholar]
- 19.Crabtree GR, Olson EN. Cell. 2002;109:S67–79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 20.Rossow CF, Minami E, Chase EG, Murry CE, Santana LF. Circ Res. 2004;94:1340–1350. doi: 10.1161/01.RES.0000128406.08418.34. [DOI] [PubMed] [Google Scholar]
- 21.Kawamura T, Ono K, Morimoto T, Akao M, Iwai-Kanai E, Wada H, Sowa N, Kita T, Hasegawa K. Circ Res. 2004;94:1492–1499. doi: 10.1161/01.RES.0000129701.14494.52. [DOI] [PubMed] [Google Scholar]
- 22.Xia Y, McMillin JB, Lewis A, Moore M, Zhu WG, Williams RS, Kellems RE. J Biol Chem. 2000;275:1855–1863. doi: 10.1074/jbc.275.3.1855. [DOI] [PubMed] [Google Scholar]
- 23.Bushdid PB, Osinska H, Waclaw RR, Molkentin JD, Yutzey KE. Circ Res. 2003;92:1305–1313. doi: 10.1161/01.RES.0000077045.84609.9F. [DOI] [PubMed] [Google Scholar]
- 24.Macian F. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 25.Hill-Eubanks DC, Gomez MF, Stevenson AS, Nelson MT. Trends Cardiovasc Med. 2003;13:56–62. doi: 10.1016/s1050-1738(02)00212-8. [DOI] [PubMed] [Google Scholar]
- 26.Dorfmuller P, Perros F, Balabanian K, Humbert M. Eur Respir J. 2003;22:358–363. doi: 10.1183/09031936.03.00038903. [DOI] [PubMed] [Google Scholar]
- 27.Nicolls MR, Taraseviciene-Stewart L, Rai PR, Badesch DB, Voelkel NF. Eur Respir J. 2005;26:1110–1118. doi: 10.1183/09031936.05.00045705. [DOI] [PubMed] [Google Scholar]
- 28.Voelkel NF, Cool C, Lee SD, Wright L, Geraci MW, Tuder RM. Chest. 1998;114:225S–230S. doi: 10.1378/chest.114.3_supplement.225s. [DOI] [PubMed] [Google Scholar]
- 29.Aramburu J, Yaffe MB, Lopez-Rodriguez C, Cantley LC, Hogan PG, Rao A. Science. 1999;285:2129–2133. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- 30.Gratton JP, Yu J, Griffith JW, Babbitt RW, Scotland RS, Hickey R, Giordano FJ, Sessa WC. Nat Med. 2003;9:357–362. doi: 10.1038/nm835. [DOI] [PubMed] [Google Scholar]
- 31.Smirnov S, Robertson T, Ward J, Aaronson P. Am J Physiol. 1994;266:H365–H370. doi: 10.1152/ajpheart.1994.266.1.H365. [DOI] [PubMed] [Google Scholar]
- 32.McKinsey TA, Olson EN. J Clin Invest. 2005;115:538–546. doi: 10.1172/JCI24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, et al. Circulation. 2006;114:1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 34.Young KA, Ivester C, West J, Carr M, Rodman DM. Am J Physiol. 2006;290:L841–L848. doi: 10.1152/ajplung.00158.2005. [DOI] [PubMed] [Google Scholar]
- 35.Michelakis ED, Hampl V, Nsair A, Wu X, Harry G, Haromy A, Gurtu R, Archer SL. Circ Res. 2002;90:1307–1315. doi: 10.1161/01.res.0000024689.07590.c2. [DOI] [PubMed] [Google Scholar]
- 36.Biswas G, Guha M, Avadhani NG. Gene. 2005;354:132–139. doi: 10.1016/j.gene.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX. Proc Natl Acad Sci USA. 2004;101:13861–13866. doi: 10.1073/pnas.0405908101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonnet S, Belus A, Hyvelin JM, Roux E, Marthan R, Savineau JP. Am J Physiol. 2001;281:L193–L201. doi: 10.1152/ajplung.2001.281.1.L193. [DOI] [PubMed] [Google Scholar]
- 39.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, Grimminger F. J Clin Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.