Abstract
IL-15 is a potent T cell chemoattractant, and this cytokine and its unique α subunits, IL-15Rα, can modify immune cell expression of several T cell chemokines and their receptors. Facial nerve axotomy in mice leads to T cell migration across an intact blood-brain-barrier (BBB), and under certain conditions T cells can provide neuroprotection to injured neurons in the facial motor nucleus (FMN). Although chemokines and chemoattractant cytokines are thought to be responsible for T cell migration to the injured cell bodies, data addressing this question are lacking. This study tested the hypothesis that T cell homing to the axotomized FMN would be impaired in knockout (KO) mice with the IL-15 and IL-15Rα genes deleted, and sought to determine if microglial responsiveness and motoneuron death are affected. Both IL-15KO and IL-15RαKO mice exhibited a marked reduction in CD3+ T cells and had fewer MHC2+ activated microglia in the injured FMN than their respective WT controls at day 14 post-axotomy. Although there was a relative absence of T cell recruitment into the axotomized FMN in both knockout strains, IL-15RαKO mice had five times more motoneuron death (characterized by perineuronal microglial clusters engulfing dead motoneurons) than their WT controls, whereas dead neurons in IL-15KO did not differ from their WT controls. Further studies are needed to dissect the mechanisms that underlie these observations (e.g., central vs. peripheral immune contributions).
IL-15 and IL-2 are members of the 4α-helix bundle family of cytokines that share the same constitutively expressed pair of signal transducing β and γ receptor subunits, but exhibit high affinity binding to unique α subunits (e.g., IL-15Rα) that confer specificity for each cytokine. Mouse microglia express IL-15 and IL-15Rα [5], and human astrocytes and neural cell lines (e.g., neuroblastoma cells) also express IL-15 mRNA [11, 20]. IL-15 is a potent T cell chemoattractant (Wilkinson and Liew, 1995). IL-15 and IL-15Rα can modify the expression of T cell chemoattractants including MCP-1, RANTES, and IP-10, chemokines and their receptors that have been implicated in autoimmune T cell infiltration of the CNS [1, 2, 8, 15].
T lymphocytes migrate in and out of the brain and other nonlymphoid organs, and can be found in very small numbers in the brain under normal physiological conditions [3, 6, 7]. Facial nerve axotomy in mice leads to T cell migration across an intact blood-brain-barrier (BBB) where they traffic to and accumulate in the facial motor nucleus (FMN) of the axotomized peripheral nerve [19]. In certain contexts, T cells can provide neuroprotection to axotomized motoneurons. Severe combined immunodeficient (SCID) and recombinase activating gene-2 (RAG-2) deficient mice, which lack functionally mature T and B lymphocytes, develop a profound loss of facial motor neurons following axotomy and the loss of regenerative capacity is time-dependently prevented when normal lymphocytes are adoptively transferred prior to axotomy[16, 23, 24]. The signals that draw T cells to the injured cell bodies in the FMN are presumed to be chemokines and chemoattractant cytokines, although experimental studies addressing this question are lacking. In rats, facial nerve transection was found to induce the expression of MCP-1 by neurons. Although the role of IL-15/15Rα in T cell chemoattraction in the brain has not been examined, IL-15 was found to be upregulated prior to T cell entry into the mouse sciatic nerve following constriction injury [10]. The present study therefore sought to test the hypothesis that T cell homing to the injured FMN following facial nerve axotomy would be impaired in IL-15 and IL-15Rα knockout mice, and to determine whether microglial responsiveness and motoneuron death are affected. Following facial nerve axotomy in mice, T lymphocyte levels and the rate of neuronal death (characterized by perineuronal microglial clusters engulfing and removing dead motoneurons) in the injured FMN peak at day 14. This time-point also allows for examination of potential interactions between the axotomy-induced responses of T cells, microglia and motoneurons. Thus, we compared IL-15 and IL-15Rα knockout mice and their respective wild-type controls at day 14 post-axotomy for differences in numbers of: 1) CD3+ T lymphocytes entering the axotomized FMN; 2) CD11b+ perineuronal microglial phagocytic clusters, a measure of motor neuron death, and; 3) MHC2+ activated microglia.
The experimental procedures were conducted as described previously by our lab (Petitto et al., 2003; Ha et al., 2006). Mice used in these experiments were cared for in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and were housed under specific pathogen-free conditions in individual microisolater cages. Breeding pairs of C57BL/6-IL15−/− knockout (IL-15KO) and C57BL/6-IL15+/+ wild type (IL-15WT) mice were obtained from Taconic [9]. Breeding pairs of B6x129-IL15Rα−/− knockout (IL-15RαKO) and B6x129S-IL15Rα+/+ wild-type (IL-15RαWT) mice were purchased from Jackson Laboratories [12]. The offspring were genotyped by the polymerase chain reaction (PCR). For genotyping, PCR was used to amplify targeted regions from the IL15KO, IL15WT, IL-15RαKO, and IL-15RαWT gene alleles. Genomic DNA was extracted from tail snips obtained shortly after weaning [17]. PCR reactions were performed using a 25 μl total reaction volume containing 1 μM each of forward and reverse primers, 0.2 mM each of dNTP, 2 mM magnissium chloride, 1.5 U Taq DNA polymerase and 0.1 μg genomic DNA with a thermal cycler (iCycler, Bio-Rad). The cycling parameters were as follows: hot start 95 °C (2 min); denaturing 94 °C (30 s); annealing 58 °C (30 s); extension 72 °C (45 s) with final extension step of 7 min. Thirty two cycles was used for these experiments. The 5′ and 3′ primers used to identify the IL15KO allele (520 bp) were 5′-GAATGGGCTGACCGCTTCCTCG and 5′-TCATATCCTCTGCACCTTGACTG. The 5′ and 3′ primers for the IL15WT allele (240 bp) were 5′-GAGGGCTAAATCTGATGCGTGTG. The 5′ and 3′ primers for IL15RαKO allele ( 280 bp) were 5′-CTTGGGTGGAGAGGCTATC and 5′-AGGTGAGATGACAGGAGATC, and the 5′ and 3′ primers for IL15RαWT allele (171 bp) were 5′-ATTGAGCATGCTGACATCCG and 5′-ACTGATGCACTTGAGGCTGG.
Adult mice were 10–12-weeks-old at the time of surgery. Animals were anesthetized with 4% isoflurane. The right facial nerve was transected at its exit from the stylomastoid foramen and sutures were applied at the incision site as described previously [4, 18]. Mice were anesthetized by intraperitoneal injection of a 0.5 mg/ml ketamine cocktail (ketamine/xylazine/acepromazine) in a 3:3:1 ratio and were perfused with 4% paraformaldehyde in phosphate buffered saline (PF/PBS) at 14 days post-axotomy [16]. Brain stems were removed and post-fixed by immersion in 4% PF/PBS for 2 hours at room temperature. Following cryoprotection by immersion in 30% sucrose overnight at 4 °C, tissue was snap frozen in isopentane (−80 °C) and stored at −80 °C. Fifteen μm coronal sections were cut at the level of facial motor nucleus in a cryostat. Sections were collected on Superfrost/Plus slides (Fisher Scientific) and stored at −80 °C. For immunohistochemistry, tissue sections were incubated in normal goat serum (Vector, 1:30 NGS/PBS) for 1–2 h at room temperature. Sections were in overnight immersion with anti-mouse CD3 (clone 17A2; PharMingen; 1:500), CD11b (clone 5C6; Serotec; 1:500), or MHC2 I-A/I-E (M5/114.15.2; PharMingen; 1:400) primary antibody at 4 °C. Visualization of the primary antibodies was performed by incubation of sections in goat anti-rat secondary antibody (1:2000, Vector Labs) for 1 h at room temperature followed by incubation of in avidin-peroxidase conjugates (1:500, Sigma) for 1 h. Sections were washed in 1 X PBS between each incubation step. The chromagen reaction was revealed by incubation in 3,3′-diaminobenzidine (DAB)-H2O2 solution (Sigma; 0.07% DAB/0.004% H2O2). Sections were counterstained with cresyl violet, dehydrated in ascending alcohol washes, cleared in xylenes, and coverslipped. Control slides using either the primary or secondary antibodies run alone did not yield a detectable signal for CD3+ T cells, CD11b+ microglial phagocytic clusters, or MHC2+ activated microglia. An average of eight sections (approximately 1/5 of the entire FMN) were used to assess the number of CD3+ T cells, CD11b+ microglial phagocytic clusters, or MHC2+ activated microglia per mouse FMN. All sections were counted blindly. Mean counts/section throughout the FMN were calculated for statistical analyses, as described previously [18].
Analysis of variance (ANOVA) was used to make comparisons between subject groups. Mice were 8–12 weeks of age and the KO mice and their respective WT controls were matched for age, and the groups were balanced for sex. For the IL-15KO (n=7) and IL-15WT (n=6) groups, all of the mice were female. For the IL-15RαKO (n=7) and IL-15RαWT (n=7) groups, the male/female ratios were 4/3 and 3/4, respectively. Using all subjects, no main effects of sex were found for any of the dependent variables. Figure 1 shows the results of quantitative assessments comparing numbers of CD3+ T cells, MHC2+ activated microglia, and CD11b+ microglial clusters engulfing dead neurons between IL-15KO and IL-15WT mice. As depicted in Figure 1, compared to IL-15WT mice, there was a relative absence of T cells in the axotomized FMN of IL-15KO mice (F[1,11]=88.3, p<0.001). IL-15KO mice also had significantly fewer MHC2+ activated microglia than IL-15WT mice (F[1,11]=8.7, p<0.01). The groups did not differ in the number of CD11b+ microglial clusters engulfing dead neurons. Figure 2 shows representative photomicrographs comparing IL-15KO and IL-15WT mice for each of these dependent variables. As seen in Figure 3, IL-15RαKO mice showed a reduction in CD3+ T cells compared to levels seen in the injured FMN of IL-15RαWT mice (F[1,12]=16.3, p<0.01). IL-15RαKO mice had significantly fewer MHC2+ activated microglia (F[1,12]=9.7, p<0.01) and markedly higher numbers of CD11b+ microglial clusters engulfing dead motor neurons (F[1,12]=14.6, p<0.01) in the injured FMN than IL-15RαWT mice.
Figure 1.
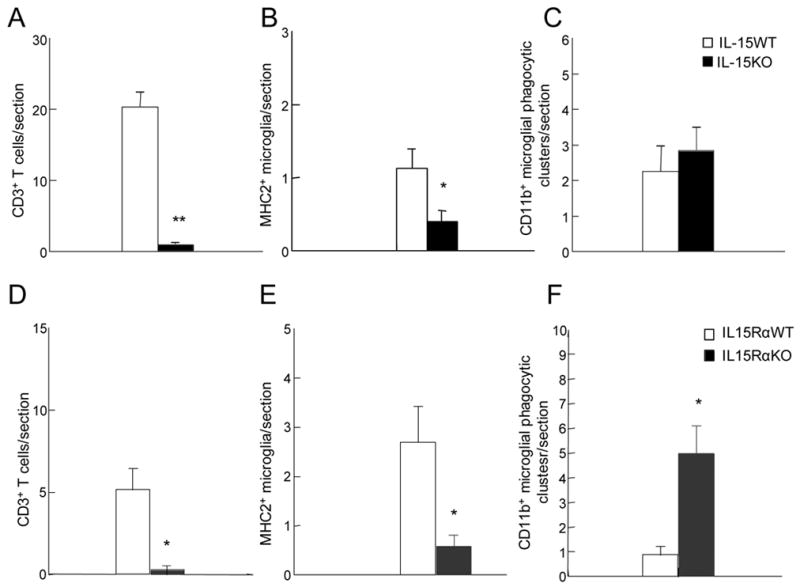
Quantification of CD3+ T cells, MHC2+ activated microglia, and CD11b+ microglial phagocytic clusters in the FMN at day 14 post-axotomy. Each bar in figures A–C represents the S.E.M. of 6 (IL-15 WT) and 7 (IL-15 KO) mice. Each bar in figures D-F represents the S.E.M. of 7 (IL-15RαWT & IL-15RαKO) mice. *p<0.01, **p<0.001.
Figure 2.
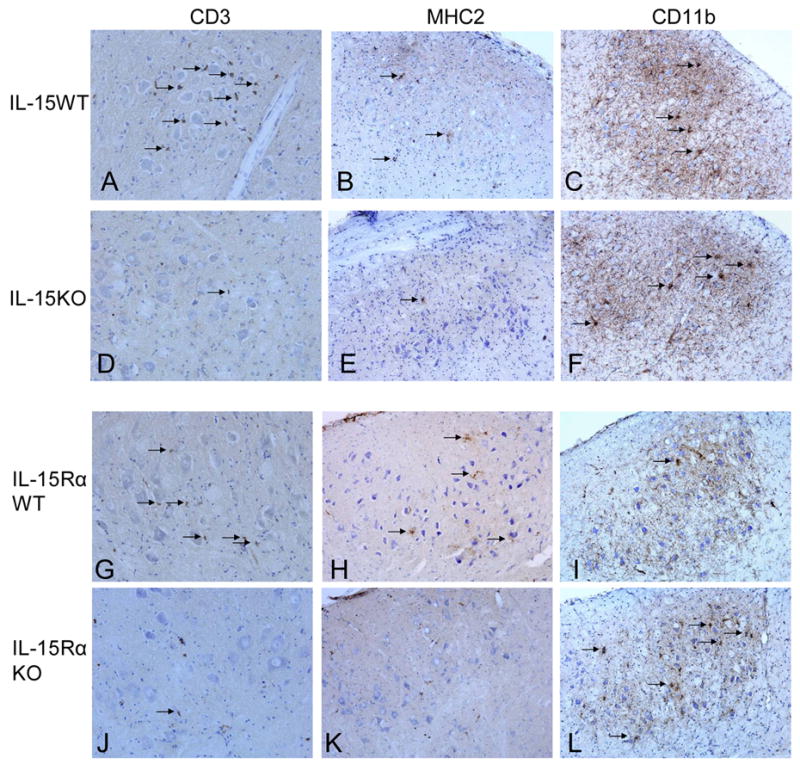
Photomicrographs of CD3+ T cells, MHC2+ activated microglia, and CD11b+ microglial phagocytic clusters in the axotomized FMN of IL-15WT, IL-15KO, IL-15RαWT, and IL-15RαKO mice.
This study confirmed our hypothesis that T cell homing to the injured FMN following facial nerve axotomy would be impaired in mice deficient in IL-15 and IL-15Rα. In both KO strains there was a marked reduction in T cells trafficking into the axotomized FMN. The most likely explanation for this impairment in the IL-15KO mice is that the loss of IL-15 in the brain results in loss of the cytokine’s potent T cell chemoattractant properties and reduced numbers of T cells in the injured FMN [26]. In the IL-15Rα-deficient mice, the absence of high affinity binding sites on T cells may impair their ability to respond to IL-15 released in response to FMN injury. Microglia can express both IL-15 and IL-15Rα genes, and loss of the receptor may lead to impaired autocrine production of IL-15 by microglia [5]. There is evidence in the immunological literature suggesting that IL-15 bound to 15Rα on donor cells (e.g., microglial cell) can be trans-presented to a recipient cell (e.g., T cell) containing the constituitively expressed signaling subunits, IL-2/IL-15Rβ and common IL-2Rγ (a common subunit for multiple cytokines including IL-2 and IL-15) [13, 21]. Thus, absence of IL-15Rα could also impair the ability of IL-15 to signal other cells in this manner. Receptors for IL-15, and its related cytokine, IL-2 share the same constitutively expressed pair of signal transducing β and γ receptor subunits. When the expression of IL-15Rα is absent, IL-2 can transmit intracellular signals to CNS cells that express the dimeric receptor complex (IL-2/IL-15Rβ and IL-2Rγ). We have shown previously that IL-2 has neurodegenerative effects on motoneurons in the axotomized FMN (Petitto et al., 2003). Thus, it is possible that IL-2’s effects may contribute to the high level of dead motoneurons seen at day 14 in the IL-15RαKO mice. It is also possible that the markedly reduced numbers of T cells in the axotomized FMN of both strains of KO mice could be associated with secondary changes in other immune processes affected by these gene deletions. The most likely candidates would be T cell chemokines that are known to be affected by IL-15/IL-15Rα expression, in particular chemoattractants MCP-1, RANTES, and IP-10 and their receptors at the site of injury in the brain [1, 2, 8, 15].
Despite the relative absence of T cells in the FMN of both of the KO strains, there were divergent effects on motoneuron death between the KO strains. Whereas the number of dead motoneurons (microglial phagocytic clusters) did not differ between IL-15KO and IL-15WT mice, IL-15RαKO mice had five times more dead neurons than 15RαWT mice. It is possible that this discrepancy could be due to the different genetic backgrounds of the two KO strains. Whereas IL-15KO mice were on the C57BL/6 (B6) background, 15RαKO were on the B6 X 129 background. These background differences would appear unlikely to be a significant contributory factor, in light of recent data from our lab using the facial nerve axotomy model where we found in side-by-side comparisons that B6 X 129 mice did not differ significantly from B6 mice in numbers of perineuronal microglial phagocytic clusters at day 14 post-axotomy [4]. It has been shown that a substantial percentage of motoneurons in the injured FMN atrophy rather than die (McPhail et al., 2004). Because these atrophied motorneurons are more difficult to detect due to their decreased ability to uptake standard counterstains (i.e., cresyl violet), assessments of dead neurons being engulfed by phagocytic microglia at 14 days post-axotomy (the peak of the rate of neuronal death following nerve axotomy) is a good measure to assess neuronal status post-injury. To get a more complete and reliable picture of neuronal status post-axotomy in these knockout mice, it will also be important in future studies to quantify the level of cumulative neuronal death at a later point in time (e.g., 4 – 5 weeks post-axotomy) by counting the surviving neurons in the axotomized FMN. Previous studies by Raivich’s lab have shown that levels of neuronal death assessed by perineuronal microglial phagocytic clusters at day 14 post-axotomy correlated with cumulative neuronal loss as assessed by cell counting at day 30 post-axotomy in mice (Raivich et al., 2002; Bohatscek et al., 2004). Nonetheless, though unlikely, it is possible that the kinetics of neuronal death assessed by micoglial phagocytic clusters could be unexpectedly altered in the IL-15RαKO mice, or that there are more detectable clusters at day 14 due to some unforeseen factor not related to neuronal death per se (e.g., the knockout somehow impairs phagocytosis so the dead neurons are not cleared normally).
Differences in the composition of peripheral T cell subsets between the two KO strains may be a significant factor that accounts for notable differences in neuronal death in the relative absence of T cells in the injured FMN. Serpe et al. showed in adoptive transfer experiments of immunodeficient mice that CD4+ T cells were the subset of T cells that were responsible for neuroprotection following facial nerve axotomy [22]. The deletion of the IL-15 gene in mice results in a significant reduction (40–50%) in CD8+ T cell numbers populating the spleen and lymph nodes, whereas CD4+ T cells are not affected [9, 25]. In addition to having reduced levels of CD8+ T cell numbers in the spleen and lymph nodes, these IL-15RαKO mice (involving exons 2–3) also have reduced levels of CD4+ T cells (by more than 50%) as well [12, 25]. Thus, it is possible that despite the absence of T cells in the injured FMN, normal CD4+ T cells in IL-15KO mice may produce (or induce other cells to produce) peripheral factors such as cytokines that provide neurotrophic support to injured facial motor neurons. In spite of these percentage reductions in T cell subsets, there are sufficient numbers of peripheral T cells available to traffick into the injured FMN. Thus, we speculate that the relative absence of T cells in the axotomized FMN of both knockout strains is due to alterations in chemoattraction. Further studies will be necessary to determine the central vs. peripheral contributions of T cell subsets on neuronal regeneration in these KO strains.
Finally, loss of IL-15Rα and IL-15 both resulted in a significant reduction of MHC2+ activated microglia in the injured FMN compared to their respective wild-type controls. Although this has not been examined before in the brain to our knowledge, the reduced MHC2+ expression by microglia in the axotomized FMN of IL-15KO and IL-15RαKO mice is consistent with findings in the immunological literature demonstrating that deletion of these genes impairs antigen-simulated MHC2+ expression and other measures of activation in peripheral dendritic cells and macrophages [14].
In summary, both IL-15KO and IL-15RαKO mice exhibited a marked reduction in CD3+ T cells and had fewer MHC2+ activated microglia in the injured FMN than their respective WT controls at day 14 post-axotomy. Although there was a relative absence of T cell recruitment into the axotomized FMN in both knockout strains, IL-15RαKO mice had five times more dead motoneurons than their WT controls, whereas dead neurons in IL-15KO did not differ from their WT controls. Further studies are needed to dissect the mechanisms that underlie these observations (e.g., central vs. peripheral immune contributions, secondary effects of these gene deletions on T cell chemokines).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Badolato R, Ponzi AN, Millesimo M, Notarangelo LD, Musso T. Interleukin-15 (IL-15) induces IL-8 and monocyte chemotactic protein 1 production in human monocytes. Blood. 1997;90:2804–2809. [PubMed] [Google Scholar]
- 2.Chen JP, Liao NS, Lai SL, Hsu L, Mao WY, Ku MC, Liao F. Reduced 2,4-dinitro-1-fluorobenzene-induced contact hypersensitivity response in IL-15 receptor alpha-deficient mice correlates with diminished CCL5/RANTES and CXCL10/IP-10 expression. European journal of immunology. 2005;35:690–698. doi: 10.1002/eji.200425577. [DOI] [PubMed] [Google Scholar]
- 3.Cose S, Brammer C, Khanna KM, Masopust D, Lefrancois L. Evidence that a significant number of naive T cells enter non-lymphoid organs as part of a normal migratory pathway. Eur J Immunol. 2006;36:1423–1433. doi: 10.1002/eji.200535539. [DOI] [PubMed] [Google Scholar]
- 4.Ha GK, Huang Z, Streit WJ, Petitto JM. Endogenous T lymphocytes and microglial reactivity in the axotomized facial motor nucleus of mice: effect of genetic background and the RAG2 gene. Journal of neuroimmunology. 2006;172:1–8. doi: 10.1016/j.jneuroim.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Hanisch UK, Lyons SA, Prinz M, Nolte C, Weber JR, Kettenmann H, Kirchhoff F. Mouse brain microglia express interleukin-15 and its multimeric receptor complex functionally coupled to Janus kinase activity. The Journal of biological chemistry. 1997;272:28853–28860. doi: 10.1074/jbc.272.46.28853. [DOI] [PubMed] [Google Scholar]
- 6.Hickey WF. Leukocyte traffic in the central nervous system: the participants and their roles. Semin Immunol. 1999;11:125–137. doi: 10.1006/smim.1999.0168. [DOI] [PubMed] [Google Scholar]
- 7.Hickey WF. Migration of hematogenous cells through the blood-brain barrier and the initiation of CNS inflammation. Brain Pathol. 1991;1:97–105. doi: 10.1111/j.1750-3639.1991.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 8.Karpus WJ, Ransohoff RM. Chemokine regulation of experimental autoimmune encephalomyelitis: temporal and spatial expression patterns govern disease pathogenesis. J Immunol. 1998;161:2667–2671. [PubMed] [Google Scholar]
- 9.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. The Journal of experimental medicine. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleinschnitz C, Hofstetter HH, Meuth SG, Braeuninger S, Sommer C, Stoll G. T cell infiltration after chronic constriction injury of mouse sciatic nerve is associated with interleukin-17 expression. Experimental neurology. 2006;200:480–485. doi: 10.1016/j.expneurol.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Lee YB, Satoh J, Walker DG, Kim SU. Interleukin-15 gene expression in human astrocytes and microglia in culture. Neuroreport. 1996;7:1062–1066. doi: 10.1097/00001756-199604100-00022. [DOI] [PubMed] [Google Scholar]
- 12.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 13.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annual review of immunology. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 14.Ohteki T, Suzue K, Maki C, Ota T, Koyasu S. Critical role of IL-15-IL-15R for antigen-presenting cell functions in the innate immune response. Nature immunology. 2001;2:1138–1143. doi: 10.1038/ni729. [DOI] [PubMed] [Google Scholar]
- 15.Perera LP, Goldman CK, Waldmann TA. IL-15 induces the expression of chemokines and their receptors in T lymphocytes. J Immunol. 1999;162:2606–2612. [PubMed] [Google Scholar]
- 16.Petitto JM, Huang Z. Cloning the full-length IL-2/15 receptor-beta cDNA sequence from mouse brain: evidence of enrichment in hippocampal formation neurons. Regulatory peptides. 2001;98:77–87. doi: 10.1016/s0167-0115(00)00229-9. [DOI] [PubMed] [Google Scholar]
- 17.Petitto JM, Huang Z, Hartemink DA, Beck R., Jr IL-2/15 receptor-beta gene deletion alters neurobehavioral performance. Brain research. 2002;929:218–225. doi: 10.1016/s0006-8993(01)03393-5. [DOI] [PubMed] [Google Scholar]
- 18.Petitto JM, Huang Z, Lo J, Streit WJ. IL-2 gene knockout affects T lymphocyte trafficking and the microglial response to regenerating facial motor neurons. Journal of neuroimmunology. 2003;134:95–103. doi: 10.1016/s0165-5728(02)00422-8. [DOI] [PubMed] [Google Scholar]
- 19.Raivich G, Jones LL, Kloss CU, Werner A, Neumann H, Kreutzberg GW. Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. J Neurosci. 1998;18:5804–5816. doi: 10.1523/JNEUROSCI.18-15-05804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satoh J, Kurohara K, Yukitake M, Kuroda Y. Interleukin-15, a T-cell growth factor, is expressed in human neural cell lines and tissues. Journal of the neurological sciences. 1998;155:170–177. doi: 10.1016/s0022-510x(97)00310-9. [DOI] [PubMed] [Google Scholar]
- 21.Schluns KS, Stoklasek T, Lefrancois L. The roles of interleukin-15 receptor alpha: trans-presentation, receptor component, or both? The international journal of biochemistry & cell biology. 2005;37:1567–1571. doi: 10.1016/j.biocel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Serpe CJ, Coers S, Sanders VM, Jones KJ. CD4+ T, but not CD8+ or B, lymphocytes mediate facial motoneuron survival after facial nerve transection, Brain, behavior. and immunity. 2003;17:393–402. doi: 10.1016/s0889-1591(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 23.Serpe CJ, Kohm AP, Huppenbauer CB, Sanders VM, Jones KJ. Exacerbation of facial motoneuron loss after facial nerve transection in severe combined immunodeficient (scid) mice. J Neurosci. 1999;19:RC7. doi: 10.1523/JNEUROSCI.19-11-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serpe CJ, Sanders VM, Jones KJ. Kinetics of facial motoneuron loss following facial nerve transection in severe combined immunodeficient mice. Journal of neuroscience research. 2000;62:273–278. doi: 10.1002/1097-4547(20001015)62:2<273::AID-JNR11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 25.Van Belle T, Grooten J. IL-15 and IL-15Ralpha in CD4+T cell immunity. Archivum immunologiae et therapiae experimentalis. 2005;53:115–126. [PubMed] [Google Scholar]
- 26.Wilkinson PC, Liew FY. Chemoattraction of human blood T lymphocytes by interleukin-15. The Journal of experimental medicine. 1995;181:1255–1259. doi: 10.1084/jem.181.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]


