Abstract
Limiting dopamine β-monooxygenase results in lower norepinephrine (NE) and higher dopamine (DA) concentrations in copper-deficient (Cu−) tissues compared to copper-adequate (Cu+) tissues. Mice and rat offspring were compared to determine the effect of differences in dietary copper (Cu) deficiency started during gestation or lactation on catecholamine, NE and DA, content in brain and heart. Holtzman rat and Hsd:ICR (CD-1) outbred albino mouse dams were fed a Cu- diet and drank deionized water or Cu supplemented water. Offspring were sampled at time points between postnatal ages 12 and 27. For both rat and mouse Cu- tissue, NE and DA changes were greater at later ages. Though Cu restriction began earlier in rats than mice in the gestational model, brain NE reduction was more severe in Cu- mice than Cu- rats. Cardiac NE reduction was similar in Cu- rodents in the gestation models. In the lactation model, mouse catecholamines were altered more than rat catecholamines. Furthermore, following lactational Cu deficiency Cu- mice were anemic and exhibited cardiac hypertrophy, Cu- rats displayed neither phenotype. Within a species, changes were more severe and proportional to the length of Cu deprivation. Lactational Cu deficiency in mice had greater consequences than in rats.
Keywords: copper-deficient, rats, mice, catecholamines, heart, brain
INTRODUCTION
Micronutrients have long been known to be necessary for adequate development. Copper (Cu) is one of these micronutrients that is essential for all biological systems and serves as a cofactor for many mammalian enzymes (Prohaska, 2006). Ewes grazing on pastures low in Cu delivered lambs suffering from neonatal ataxia (Bennetts and Chapman, 1937). Guinea pigs born to copper-deficient (Cu−) dams have major reductions in cerebellar size (Everson et al., 1967). Rat pups nursed by Cu- dams exhibit focal necrosis of the brain and other neural lesions (Carlton and Kelly, 1969). Furthermore, embryonic lethality was the outcome of genetic studies in which the copper transporter, Ctr1, was deleted in mice (Kuo et al., 2001; Lee et al., 2001). Brain Cu status was altered even in heterozygotes. These data clearly demonstrate the need for copper during brain development.
Mammals contain the enzyme dopamine β-monooxygenase (DBM) (EC 1.14.17.1) which is dependent on Cu to convert dopamine (DA) into norepinephrine (NE) (Friedman and Kaufman, 1965). Deletion of this gene in mice leads to embryonic lethality that can be rescued by exogenous NE administration (Thomas et al., 1995). Limitation in DBM following dietary Cu deficiency was first demonstrated in heart of Cu- rats (Missala et al., 1967). Lower NE steady state levels were first shown for Cu- rat brain (Prohaska and Wells, 1974). Shortly thereafter limitation in DBM was reported in Cu- lambs (O'Dell et al., 1976). DBM limitation has also been reported for Cu- cattle (Hesketh, 1981). DBM limitation has been demonstrated in humans with genetic copper deficiency, Menkes’ disease, due to deletion of the copper efflux transporter ATP7A (Grover et al., 1982). Mice with mutation and loss of function in this same gene have lower brain NE (Hunt, 1974). Thus, DBM function in brain and heart require adequate Cu. Previous work in our lab has demonstrated that limitation of dietary Cu later in development in mice and rats limits DBM and results in NE depression and DA elevation in heart and brain tissue (Prohaska et al., 1990).
Interestingly, experiments in rats that were copper-deficient during development but then repleted with Cu for several months demonstrated altered auditory startle response (Prohaska and Hoffman, 1996) and impaired coordination behavior (Penland and Prohaska, 2004). While no definitive neurochemical link has been established for the changes in behavior it is possible that a catecholamine imbalance during development is responsible for these outcomes.
Mice and rats have different thresholds of sensitivity to Cu deficiency. Our lab demonstrated that when mouse dams were placed on the Cu- diet at embryonic day 13 (E13) pups did not survive to weaning and died midway through lactation (Prohaska and Brokate, 2002). When mouse dams were placed on the diet at E19, all the pups survived through weaning. The reason for lack of survival is not known but could be related to altered concentrations of the neurotransmitters, NE and DA. Rats seem to be less sensitive to Cu deprivation. Dams can be started on the Cu- diet throughout gestation or at E7 and pups survive past weaning (Prohaska and Wells, 1974; Prohaska and Bailey, 1994). Comparison of sensitivity to Cu deprivation between the two species may be helpful in planning future models that examine the effects of Cu deficiency during development.
Previous work examined brain catecholamine concentrations in Cu- rat and mouse models, however, these studies were conducted using whole brain (Prohaska and Smith, 1982). This masked changes in DA due to regional brain differences. Studies using embryonic Cu restriction examined multiple brain regions but did not include heart tissue (Prohaska and Bailey, 1993b, 1994). Current experiments were designed to compare the impact of Cu deficiency on catecholamine concentrations in rat and mouse models starting at two different time points (gestation or lactation) and in two different organs (heart and medulla/pons (a brain region that reflects DBM function)). Catecholamine changes have not previously been conducted on tissues from rodents only restricted during lactation.
MATERIALS AND METHODS
Animal Care and Diets
Holtzman rats (Rattus norvegicus) and Hsd:ICR (CD-1) outbred albino mice (Mus musculus) were purchased commercially (Harland Sprague Dawley, Indianapolis, IN, USA) and bred for the experiments. Animals were fed a Cu- diet (Teklad Laboratories, Madison, WI, USA), similar to the AIN-76A diet and contained the following major components (g/kg diet): sucrose, 500; casein, 200; cornstarch, 150; corn oil, 50; cellulose, 50; modified AIN-76 mineral mix, 35; AIN-76A vitamin mix, 10. Cupric carbonate was omitted from the AIN-76A mineral mix in the Cu- diet. It contained 0.36 mg Cu/kg and 53 mg Fe/kg by chemical analysis. Cu+ animals drank deionized water with copper sulfate (20mg Cu/L) and Cu- animals drank deionized water.
Onset of Cu deprivation was started at two different times, during gestation or beginning at parturition (lactation only). Due to differences in Cu sensitivity it was necessary to start mice later in gestation than rats. Mouse dams were placed on the treatment at E17, the earliest date that allowed survival to weaning in the Cu- group, and were continued on this treatment throughout lactation. This was the gestation model (G). Rats in the G model were placed on the treatment at E7. These procedures are similar to established recent protocols (Prohaska and Broderius, 2006). Rat and mouse dams in the lactational (L) model were placed on treatments at parturition. On postnatal day 2 (P2), litter sizes were culled to ten pups. All animals had free access to diet and drinking water and were maintained at 24°C with 55% relative humidity on a 12-hour light cycle (0700–1900 h light). All protocols were approved by the University of Minnesota Animal Care Committee.
Male mice were sampled during suckling (S) at P12 and postweanling (PW) at P27. Male rats were also sampled at two times during S at P13 or P14 and PW at ages P 21 or P24. Thus, we studied two species (mice and rats), two diet treatments (Cu− and Cu+), two time models (G and L), and two ages (S and PW). After weighing, all animals were lightly anesthetized with diethyl ether and killed by decapitation. Brains, livers, hearts, and heparinized blood were harvested from both mice and rats. Tissues were weighed and either processed for biochemical analysis or frozen in liquid nitrogen and stored at −80°C until use. Brains were dissected on a chilled glass plate and the medulla/pons was separated from the rest of the brain for catecholamine analysis and the remainder was digested for Cu content.
Biochemical Analysis
Hemoglobin was determined spectrophotometrically as metcyanhemoglobin (Prohaska, 1983). Plasma was obtained and the activity of the cuproprotein ceruloplasmin (EC 1.16.3.1) was measured by following oxidation of o-dianisidine at 37°C (Prohaska, 1991). Brains, livers, and diet were wet-digested with concentrated HNO3 (Trace Metal Grade, Fischer Scientific, Pittsburg, PA, USA) and residue was brought to 4.0 ml with 0.1 M HNO3. Samples were analyzed for Cu by flame atomic absorption spectroscopy (model 1100 B, Perkin-Elmer, Wellesley, MA, USA).
Catecholamine Analysis
Catecholamines were extracted from tissues following homogenization with perchloric acid and underwent analysis directly or were frozen at −20°C (Pyatskowit and Prohaska, 2005). NE and DA separation was achieved using reverse-phase ion-pairing HPLC with electrochemical detection. The mobile phase was 0.25 mM 1-octanesulfonic acid, 0.1mM EDTA, and 0.1M NaH2PO4 with 4% methanol and adjusted to pH 3 with phosphoric acid (Teman and Prohaska, 1999). Samples were injected with the aid of an autosampler (SP8880, Spectra Physics, USA) onto a 3.2 x 15-mm cartridge guard column (Aquapore ODS 7 μM, Brownlee, Perkin Elmer) and a 4.6 x 250-mm analytical column (ODS II 5 μM, Regis, Morton Grove, IL, USA ). A column heater (BioAnalytical Systems (BAS), West Lafayette, IN, USA LC-22A) set at 32°C warmed the sample prior to detection. Amperometric detection (BAS LC-4B) utilized a glass-carbon electrode set at 0.7 V versus Ag/AgCl in a flow cell housing (BAS 17A). Output was recorded and integrated (Peak Simple Chromatography Data Systems, Las Vegas, NV, USA).
Statistical Analysis
Data were analyzed by factorial ANOVA (model, age, and treatment within a species) and significant interactions were compared by Fisher PLSD test, α =0.05. Due to age differences between mice and rats at sampling times only qualitative comparisons were made between species. Data were analyzed with the aid of Statview 4.5 (Abacus Concepts, Berkeley, CA, USA).
RESULTS
Mouse Biochemical Indices
Assessment of Cu status in the mouse experiment involved measurement of five indices that were analyzed by factorial ANOVA: bodyweight, liver Cu, anemia (hemoglobin), brain Cu, and cardiac hypertrophy (heart/body weight). Body weights were only affected by age. Liver Cu concentration showed a significant effect of diet and an interaction between diet and age (Fig. 1A). Cu+ S mice in both models had the highest liver Cu concentration. Though liver Cu concentration fell as mice aged, all Cu+ mice had higher concentrations of liver Cu than Cu- mice. Hemoglobin concentration demonstrated a significant interaction between diet, age, and treatment (Fig. 1C). In Cu+ mice, hemoglobin concentrations rose with age. At the S age, Cu- G mice had lower hemoglobin concentrations than Cu- L mice, but this disappeared in the older Cu- mice. Within the L model, Cu- PW mice had lower hemoglobin concentrations than Cu- S mice.
Fig. 1.
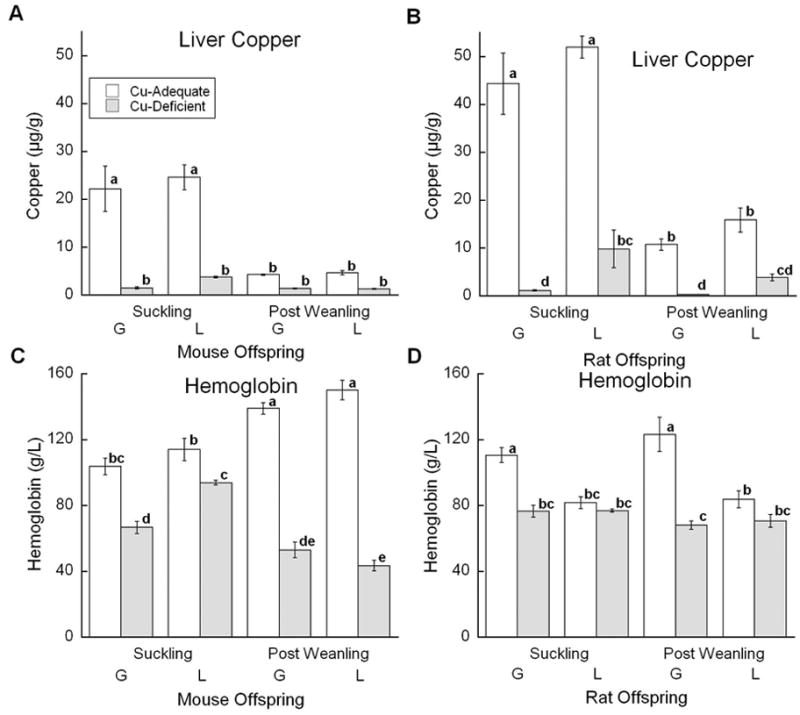
Effects of Cu deficient dietary treatment during G or L on liver Cu and hemoglobin. (A) and (C) Suckling mice at P12 and Post Weanling at P27 were sampled. Each bar represents the mean ± SEM (n=4–7). (B) and (D) Suckling rats at P13 or P14 and Post Weanling at P21 or P24 were sampled. Each bar represents the mean ± SEM (n=4). Significantly different values are denoted by unlike lower case letters. Data were analyzed by factorial ANOVA and significant interactions were compared by Fisher’s PLSD, α=0.05.
Whole brain Cu was measured to assess degree of neuronal Cu deficiency. In mice, an interaction between diet and age and between age and treatment occurred (Fig. 2A). All Cu- mice had lower brain Cu levels than Cu+ mice as expected; in control mice brain Cu increased with age. At suckling, Cu- L mice had higher brain Cu than Cu- G mice. Cu status of heart was assessed by measuring degree of cardiac hypertrophy (heart weight/ body weight). This ratio showed an interaction between diet and age (Fig. 2C). Cardiac hypertrophy increased with age in Cu- mice in both models, and was evident in all comparisons except the L model at the S age. Ceruloplasmin diamine oxidase activity was 95% lower in plasma of Cu- mice (data not shown).
Fig. 2.
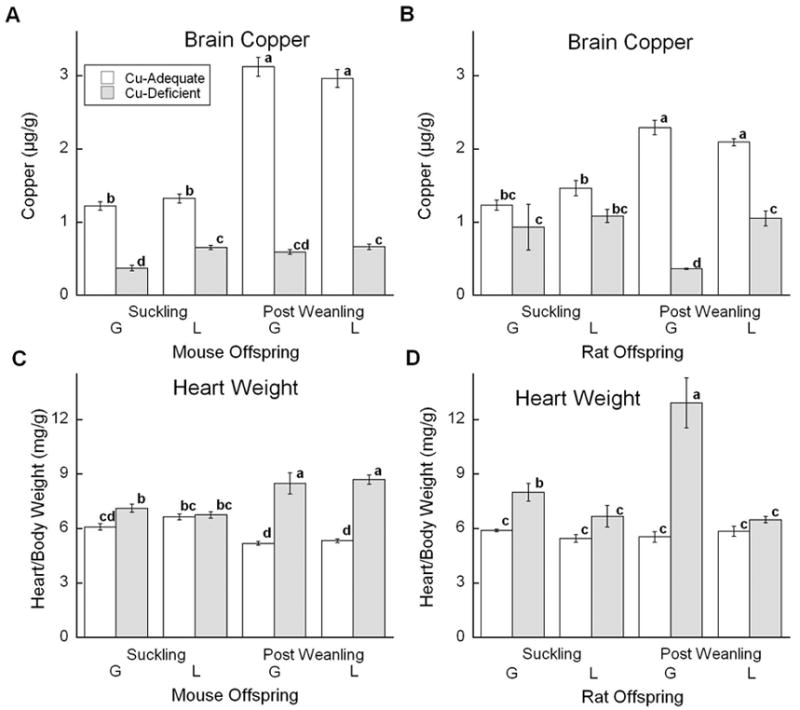
Effects of Cu deficient dietary treatment during G or L on brain Cu and cardiac hypertrophy (heart weight/body weight). (A) and (C) Suckling mice at P12 and Post Weanling at P27 were sampled. Each bar represents the mean ± SEM (n=4–7). (B) and (D) Suckling rats at P13 or P14 and Post Weanling at P21 or P24 were sampled. Each bar represents the mean ± SEM (n=4). Significantly different values are denoted by unlike lower case letters. Data were analyzed by factorial ANOVA and significant interactions were compared by Fisher’s PLSD, α =0.05.
Mouse Catecholamines
Assessment of DBM function involved measurement of NE and DA by HPLC quantification. Brain NE concentration showed a significant interaction between diet, age, and model (Fig. 3A). Reduction in NE concentrations in the PW Cu- mice was similar in both models whereas in younger mice reduction was only detected in the Cu- G mice. Analysis of brain DA concentration also showed an interaction between diet, age, and model (Fig. 3C). Brain DA was markedly elevated in half of the groups. Once again the least impacted group was the L model at the S age.
Fig. 3.
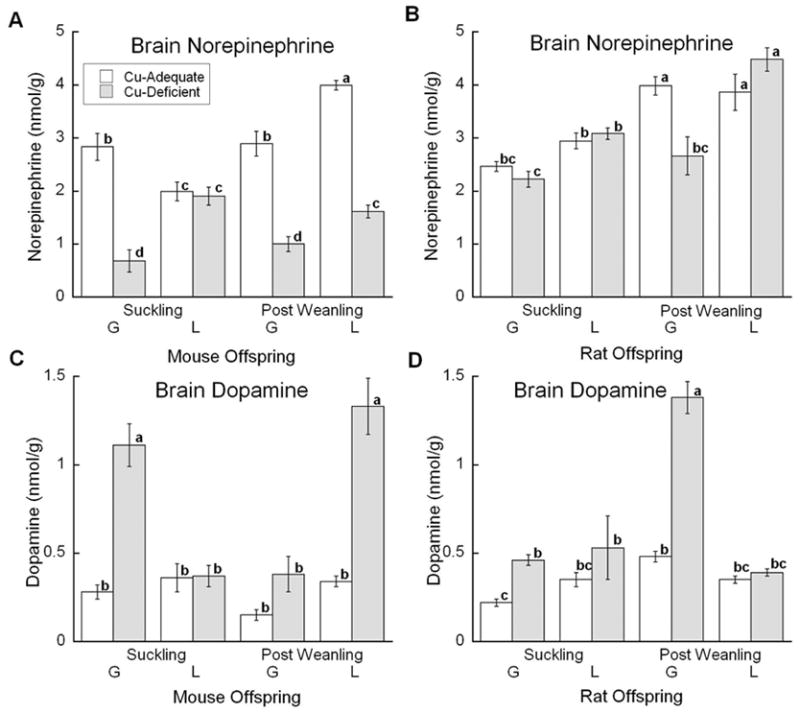
Effects of Cu deficient dietary treatment during G or L on medulla/pons (brain) NE and DA levels. (A) and (C) Suckling mice at P12 and Post Weanling at P27 were sampled. Each bar represents the mean ± SEM (n=4–7). (B) and (D) Suckling rats at P13 or P14 and Post Weanling at P21 or P24 were sampled. Each bar represents the mean ± SEM (n=4). Significantly different values are denoted by unlike lower case letters. Data were analyzed by factorial ANOVA and significant interactions were compared by Fisher’s PLSD, α =0.05.
Treatment effects on heart DBM function were also assessed using HPLC quantitiation (Fig. 4A and 4C). For heart catecholamines, an interaction between diet, age, and model was observed. Once again, mice in the L model age S were the only group unaffected in both NE and DA concentrations. However, at the older PW age, the DBM effect was clearly visible in the L model. In general, cardiac tissue from Cu- mice showed marked reduction in NE levels and major elevation in DA levels consistent with impairment in DBM function.
Fig. 4.
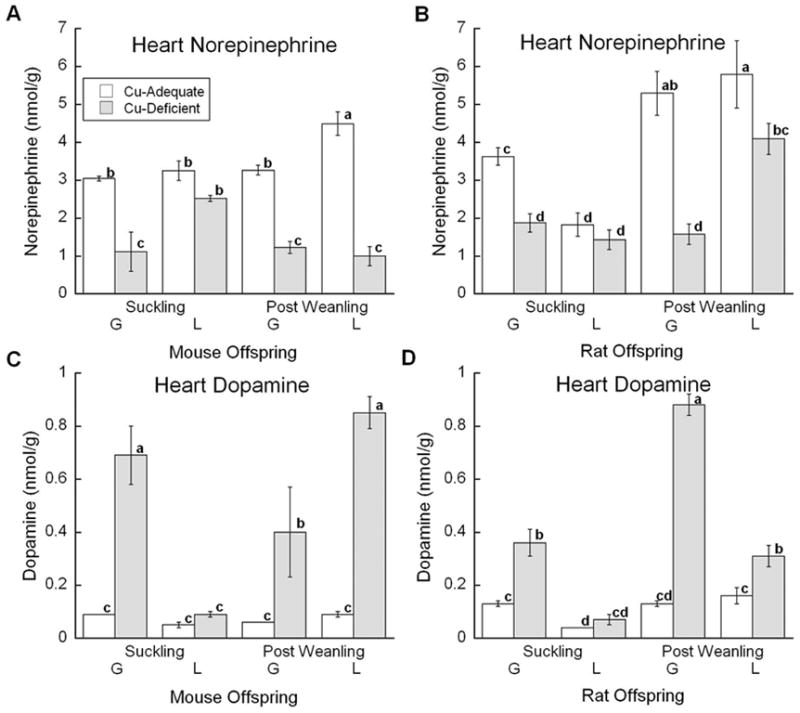
Effects of Cu deficient dietary treatment during G or L on heart NE and DA levels. (A) and (C) Suckling mice at P12 and Post Weanling at P27 were sampled. Each bar represents the mean ± SEM (n=4–7). (B) and (D) Suckling rats at P13 or P14 and Post Weanling at P21 or P24 were sampled. Each bar represents the mean ± SEM (n=4). Significantly different values are denoted by unlike lower case letters. Data were analyzed by factorial ANOVA and significant interactions were compared by Fisher’s PLSD, α =0.05.
Rat Biochemical Indices
To assess dietary treatment in the rat experiment, the same five indices of copper status measured in mice were measured and analyzed by factorial ANOVA (Fig. 1 and 2). Body weights of Cu- rats were lower than Cu+ rats (data not shown). Liver Cu concentrations showed a significant interaction between diet and age similar to mice (Fig. 1B). Cu- rats had lower Cu concentrations than Cu+ rats. As for mice, S age rats had higher liver Cu than PW age rats. At both ages, Cu- G rats had more severe reductions in Cu levels than age matched Cu- L rats. Hemoglobin analysis showed a diet by model interaction (Fig. 1D). Anemia, as judged by hemoglobin concentration, was only evident in the Cu- G groups.
Brain Cu concentrations in the rats showed a significant interaction between diet, age, and model (Fig. 2B). Brain Cu increased with age in Cu+ rats. The greatest impact of Cu deficiency was observed in the G PW rats although Cu concentration appeared lower in all Cu- groups. Degree of cardiac hypertrophy showed a significant interaction between diet, age, and model (Fig. 2D). Hypertrophy was only detected in Cu- G rats and was markedly higher in the older Cu- PW rats. Ceruloplasmin diamine oxidase activity was lower by more than 95% in plasma of Cu- rats (data not shown).
Rat Catecholamines
Brain NE concentrations showed a significant interaction between diet, age, and model (Fig. 3B). In the controls, NE increased with age in both models. Lower NE was only detected in Cu- G PW rats. This same Cu- group also showed a robust elevation in brain DA concentration (Fig. 3D). Younger Cu- G rats showed a modest increase in brain DA, but Cu- L rats at both ages did not exhibit elevated brain DA.
NE concentration in the heart showed significant interaction between diet, age and model (Fig. 4B). Cu- rats with the exception of the L model S age rats, had significantly lower concentrations of NE than control rats. The reduction in NE of the Cu- G group was greater than the Cu- L model rats at the PW age. DA concentrations showed a significant interaction between diet, age, and model (Fig. 4D). Once again, the largest increase was observed in the older Cu- G rats and the younger Cu- L models did not show any significant elevation in DA concentration.
DISCUSSION
Dietary Cu deprivation during gestation and lactation in the current experiments produced a phenotype of severe Cu deficiency. In general, the degree of Cu deficiency observed was related to the onset of Cu- treatment. This observation was made previously in another strain of mice (Prohaska and Bailey, 1993a). Perhaps the most striking observation from current experiments was the difference between mice and rat responses when Cu restriction began during lactation. For example, in the postweanling pups there was a 71% reduction of hemoglobin in Cu- compared to Cu+ mice but no change in the rats (Fig. 1). Cu- mice had a 78% reduction in brain Cu while reduction in comparable Cu- rats was only 50% (Fig. 2). Cu- mice had a 63% increase in cardiac hypertrophy while in comparable rats there was no statistical difference. Brain NE decreased 60% and brain DA increased 287% in Cu- mice but there was no significant change in rat brain catecholamines (Fig. 3). Cu- mice had a 78% reduction in heart NE compared to only a 29% decrease in rats. The increase in Cu- mouse heart DA was 849% compared to a 99% increase Cu- rats (Fig. 4). Thus, by many criteria lactational Cu deficiency is much more severe in mice compared to rats. Are humans more like rats or mice? This is an important issue to consider when selecting a nutritional model to reflect human biology.
The reason for a difference in sensitivity to lactational Cu deficiency between mice and rats could easily be due to a different requirement for Cu during suckling. However, it may be related to the degree of Cu deficiency in milk. It has recently become appreciated how remarkable the change in Cu metabolism is during lactation. For example in rats, normally most absorbed Cu is first taken up by liver but when lactation occurs Cu is diverted to the mammary gland, presumably for milk Cu excretion (Donley et al., 2002). Cu deficiency in mice reduced milk Cu content by 85% (Prohaska, 1989). Studies by others showed a quite similar reduction in rat milk from Cu deficient dams (Cohen et al., 1985). The average Cu content of milk reported in those two studies in control rodents was similar and ranged between 2–3 μg Cu/g. Further research will be necessary to fully understand the species sensitivity to Cu deprivation.
Hemoglobin values in the Cu+ rat L model seemed lower than Cu+ G model, although occasionally lower values are observed in G model offspring. In an earlier study Cu+ P22 pups had mean hemoglobin values of 80 g/L (Prohaska and Brokate, 1999). In general, hemoglobin reduction in Cu- mice was greater than in rats. This was especially true in the L model. Hemoglobin values in older Cu- mice were less than 53 g/L in both L and G groups compared to 68 and 70 g/L for Cu- rats. By comparison to Cu+ controls, the % reduction in hemoglobin was much greater in mice than rats. Since both mice and rats continue to be used to study the copper-iron interaction and anemia, model choice is an issue (Chen et al., 2006; Reeves and DeMars, 2006).
Both time models produced reductions in Cu concentration in tissues of both rodents due to Cu deficiency. Liver Cu concentrations change with age with suckling rodents having much higher liver Cu than weanling rodents (Terao and Owen, 1977). Our data in both mice and rats confirm this prior observation. In contrast to hemoglobin changes noted above, Cu deficiency had about the same impact in mice and rats for reduction in liver Cu concentration.
Unlike liver, brain Cu rose with age. This was observed previously in rats by our lab and others (Kofod, 1970; Prohaska and Wells, 1974). Our current data also show this phenomena comparing suckling (P12) versus postweanling (P27) aged mice. In younger mice at age P3, brain Cu was even lower averaging 0.86±0.03 μg/g (N=4). Reduction in brain Cu was not greatly different between Cu- rats and mice.
A cardinal feature of Cu- rats is cardiac hypertrophy (Dallman and Goodman, 1970). In our experiments, cardiac hypertrophy increased in Cu- rodents with age. In mice, both models developed hypertrophy over time whereas in rats only the G rats developed enlarged hearts. This emphasizes again the sensitivity of mice to lactational Cu deficiency. The molecular signals for this cardiac hypertrophy are still not known.
Early work on brain catecholamines failed to totally evaluate DBM limitation during Cu deficiency because whole brain was used (Feller and O'Dell, 1980; Prohaska and Smith, 1982). Later work demonstrated the medulla/pons to be a brain region reflective of active DBM function in both mice and rats (Prohaska and Bailey, 1993b, 1994). Consistent with hemoglobin and cardiac hypertrophy results, reduction in brain NE in the L model was only seen in Cu- mice as was marked elevation in brain DA.
Cardiac tissue is highly innervated with sympathetic nerves containing NE. Thus, this tissue is also highly dependent on DBM for the conversion of DA to NE. This was demonstrated using radiolabeled DA and measuring the amount of radiolabeled NE produced in Cu deficient rat heart (Missala et al., 1967). Later work in Cu deficient postweanling mice and rat hearts showed decreased NE concentrations and elevated DA concentrations confirming the need for adequate Cu for DBM function (Prohaska et al., 1990). Cardiac DBM activity in our rodents was also impaired. Using the ratio of NE/DA to compare efficiency of DBM, Cu+ L mice had values of 49.9 while the ratio for Cu- L mice was 1.2. In rats, the Cu+ L value was 37.1 and the Cu- L value was 13.2. This indicates that Cu- rodents have a greater pool of DA that hasn’t been converted to NE. Once again in the L model, Cu- mice had greater heart catecholamine alterations than the Cu- rats. It is not clear whether alterations in heart catecholamines are causally related to cardiac hypertrophy.
In both mice and rats, Cu deficiency can be induced. The degree of Cu deficiency developed depends on the species, rat or mouse, and the starting time of the Cu deprivation. In mice, the onset of Cu restriction seems to be less important than in rats. Rats needed two more weeks of Cu restriction to develop some of the hallmarks of Cu deficiency. Strain difference could also impact the Cu status and sensitivity to Cu deprivation. The difference in Cu deficiency severity seen in the two species may be beneficial when modeling perinatal or postnatal Cu deficiency in humans.
Acknowledgments
This study was supported by NIH grants HD 39708 and 2R25-GM53421-03A1. Technical assistance of Margaret Broderius and Anna Gybina is greatly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bennetts HW, Chapman FE. Copper deficiency in sheep in Western Australia: A preliminary account of the etiology of enzootic ataxia of lambs and an anemia of ewes. Aust Vet J. 1937;13:138–149. [Google Scholar]
- Carlton WW, Kelly WA. Neural lesions in the offspring of female rats fed a copper-deficient diet. J Nutr. 1969;97:42–52. doi: 10.1093/jn/97.1.42. [DOI] [PubMed] [Google Scholar]
- Chen H, Huang G, Su T, Gao H, Attieh ZK, McKie AT, Anderson GJ, Vulpe CD. Decreased hephaestin activity in the intestine of copper-deficient mice causes systemic iron deficiency. J Nutr. 2006;136:1236–1241. doi: 10.1093/jn/136.5.1236. [DOI] [PubMed] [Google Scholar]
- Cohen NL, Keen CL, Hurley LS, Lonnerdal B. Determinants of copper-deficiency anemia in rats. J Nutr. 1985;115:710–725. doi: 10.1093/jn/115.6.710. [DOI] [PubMed] [Google Scholar]
- Dallman PR, Goodman JR. Enlargement of mitochondrial compartment in iron and copper deficiency. Blood. 1970;35:496–505. [PubMed] [Google Scholar]
- Donley SA, Ilagan BJ, Rim H, Linder MC. Copper transport to mammary gland and milk during lactation in rats. Am J Physiol Endocrinol Metab. 2002;283:E667–E675. doi: 10.1152/ajpendo.00115.2002. [DOI] [PubMed] [Google Scholar]
- Everson GJ, Tsai HC, Wang TI. Copper deficiency in the guinea pig. J Nutr. 1967;93:533–540. doi: 10.1093/jn/93.4.533. [DOI] [PubMed] [Google Scholar]
- Feller DJ, O'Dell BL. Dopamine and norepinephrine in discrete areas of the copper-deficient rat brain. J Neurochem. 1980;34:1259–1263. doi: 10.1111/j.1471-4159.1980.tb09968.x. [DOI] [PubMed] [Google Scholar]
- Friedman S, Kaufman S. 3,4-dihydroxyphenylethylamine β-hydroxylase. Physical properties, copper content, and role of copper in the catalytic activity. J Biol Chem. 1965;240:4763–4773. [PubMed] [Google Scholar]
- Grover WD, Henkin RI, Schwartz M, Brodsky N, Hobdell E, Stolk JM. A defect in catecholamine metabolism in kinky-hair disease. Ann Neurol. 1982;12:263–266. doi: 10.1002/ana.410120309. [DOI] [PubMed] [Google Scholar]
- Hesketh JE. The effect of nutritional copper deprivation on the catecholamine content and dopamine-β-hydroxylase activity of rat and cattle adrenal glands. Gen Pharmacol. 1981;12:445–449. doi: 10.1016/0306-3623(81)90068-9. [DOI] [PubMed] [Google Scholar]
- Hunt DM. Primary defect in copper transport underlies mottled mutants in the mouse. Nature. 1974;249:852–854. doi: 10.1038/249852a0. [DOI] [PubMed] [Google Scholar]
- Kofod B. Iron, copper, and zinc in rat brain. Eur J Pharmacol. 1970;13:40–45. doi: 10.1016/0014-2999(70)90179-2. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Zhou B, Cosco D, Gitschier J. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc Natl Acad Sci USA. 2001;98:6836–6841. doi: 10.1073/pnas.111057298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Prohaska JR, Thiele DJ. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc Natl Acad Sci USA. 2001;98:6842–6847. doi: 10.1073/pnas.111058698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missala K, Lloyd K, Gregoriads G, Sourkes TL. Conversion of 14C-dopamine to cardiac 14C-noradrenaline in the copper- deficient rat. Eur J Pharmacol. 1967;1:6–10. doi: 10.1016/0014-2999(67)90058-1. [DOI] [PubMed] [Google Scholar]
- O'Dell BL, Smith RM, King RA. Effect of copper status on brain neurotransmitter metabolism in the lamb. J Neurochem. 1976;26:451–455. doi: 10.1111/j.1471-4159.1976.tb01495.x. [DOI] [PubMed] [Google Scholar]
- Penland JG, Prohaska JR. Abnormal motor function persists following recovery from perinatal copper deficiency in rats. J Nutr. 2004;134:1984–1988. doi: 10.1093/jn/134.8.1984. [DOI] [PubMed] [Google Scholar]
- Prohaska JR. Changes in tissue growth, concentrations of copper, iron, cytochrome oxidase and superoxide dismutase subsequent to dietary or genetic copper deficiency in mice. J Nutr. 1983;113:2048–2058. doi: 10.1093/jn/113.10.2048. [DOI] [PubMed] [Google Scholar]
- Prohaska JR. Effect of diet on milk copper and iron content of normal and heterozygous brindled mice. Nutr Res. 1989;9:353–356. [Google Scholar]
- Prohaska JR. Changes in Cu, Zn-superoxide dismutase, cytochrome c oxidase, glutathione peroxidase and glutathione transferase activities in copper- deficient mice and rats. J Nutr. 1991;121:355–363. doi: 10.1093/jn/121.3.355. [DOI] [PubMed] [Google Scholar]
- Prohaska JR. Copper. In: Bowman BA, Russell RM, editors. Present Knowledge In Nutrition. Vol. 1. International Life Sciences Institute; Washington, DC: 2006. pp. 458–470. [Google Scholar]
- Prohaska JR, Bailey WA. Copper deficiency during neonatal development alters mouse brain catecholamine levels. Nutr Res. 1993a;13:331–338. [Google Scholar]
- Prohaska JR, Bailey WR. Persistent regional changes in brain copper, cuproenzymes and catecholamines following perinatal copper deficiency in mice. J Nutr. 1993b;123:1226–1234. doi: 10.1093/jn/123.7.1226. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Bailey WR. Regional specificity in alterations of rat brain copper and catecholamines following perinatal copper deficiency. J Neurochem. 1994;63:1551–1557. doi: 10.1046/j.1471-4159.1994.63041551.x. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Bailey WR, Gross AM, Korte JJ. Effect of dietary copper deficiency on distribution of dopamine and norepinephrine in mice and rats. J Nutr Biochem. 1990;1:149–154. doi: 10.1016/0955-2863(90)90015-d. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Broderius M. Plasma peptidylglycine alpha-amidating monooxygenase (PAM) and ceruloplasmin are affected by age and copper-status in rats and mice. Comp Biochem Physiol Part B. 2006;143:360–366. doi: 10.1016/j.cbpb.2005.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohaska JR, Brokate B. Copper deficiency alters rat dopamine beta-monooxygenase mRNA and activity. J Nutr. 1999;129:2147–2153. doi: 10.1093/jn/129.12.2147. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Brokate B. The timing of perinatal copper deficiency in mice influences offspring survival. J Nutr. 2002;132:3142–3145. doi: 10.1093/jn/131.10.3142. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Hoffman RG. Auditory startle response is diminished in rats after recovery from perinatal copper deficiency. J Nutr. 1996;126:618–627. doi: 10.1093/jn/126.3.618. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Smith TL. Effect of dietary or genetic copper deficiency on brain catecholamines, trace metals and enzymes in mice and rats. J Nutr. 1982;112:1706–1717. doi: 10.1093/jn/112.9.1706. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Wells WW. Copper deficiency in the developing rat brain: a possible model for Menkes' steely-hair disease. J Neurochem. 1974;23:91–98. doi: 10.1111/j.1471-4159.1974.tb06920.x. [DOI] [PubMed] [Google Scholar]
- Pyatskowit JW, Prohaska JR. L-threo 3,4-dihydroxyphenylserine treatment during mouse perinatal and rat postnatal development does not alter the impact of dietary copper deficiency. Nutr Neurosci. 2005;8:173–181. doi: 10.1080/10284150500097182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves PG, DeMars LC. Signs of iron deficiency in copper-deficient rats are not affected by iron supplements administered by diet or by injection. J Nutr Biochem. 2006;17:635–642. doi: 10.1016/j.jnutbio.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Teman PT, Prohaska JR. Copper-deficient rats have altered plasma dihydroxyphenylacetic acid and spleen catecholamine levels. Nutr Neurosci. 1999;2:103–111. doi: 10.1080/1028415X.1999.11747268. [DOI] [PubMed] [Google Scholar]
- Terao T, Owen CA., Jr Copper metabolism in pregnant and pospartum rat and pups. Am J Physiol. 1977;232:E172–179. doi: 10.1152/ajpendo.1977.232.2.E172. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]


