Abstract
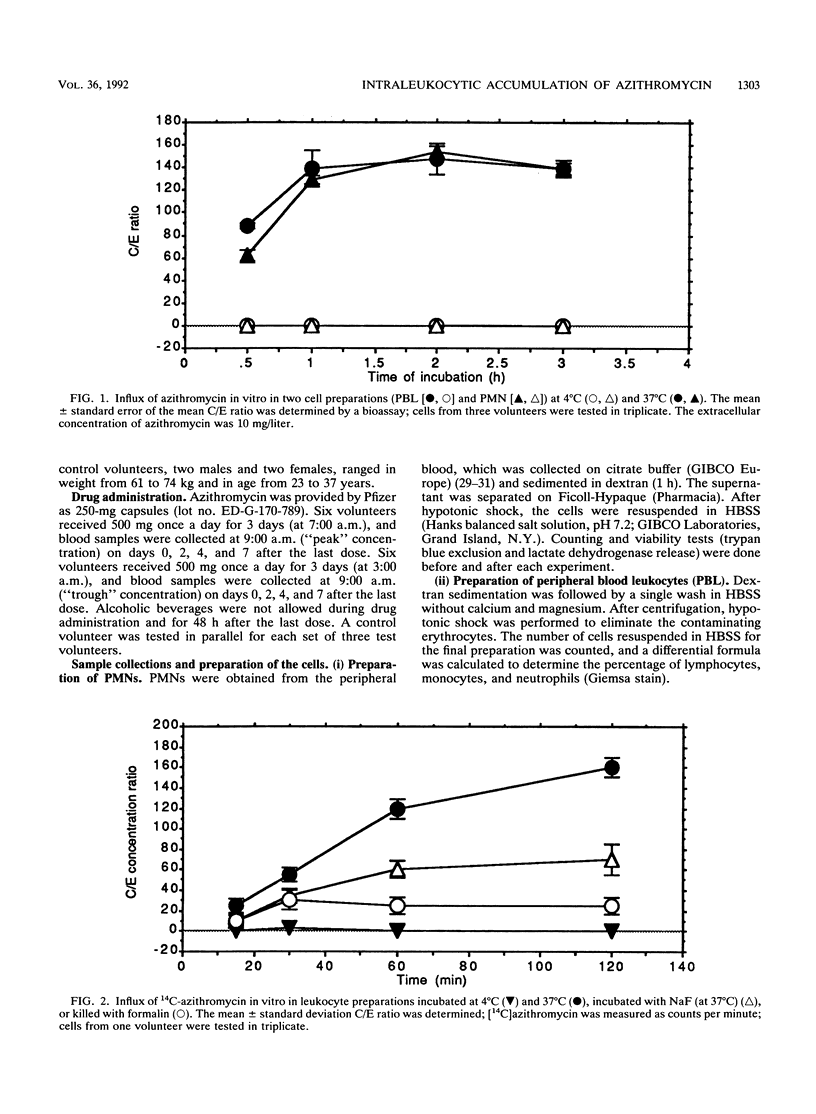
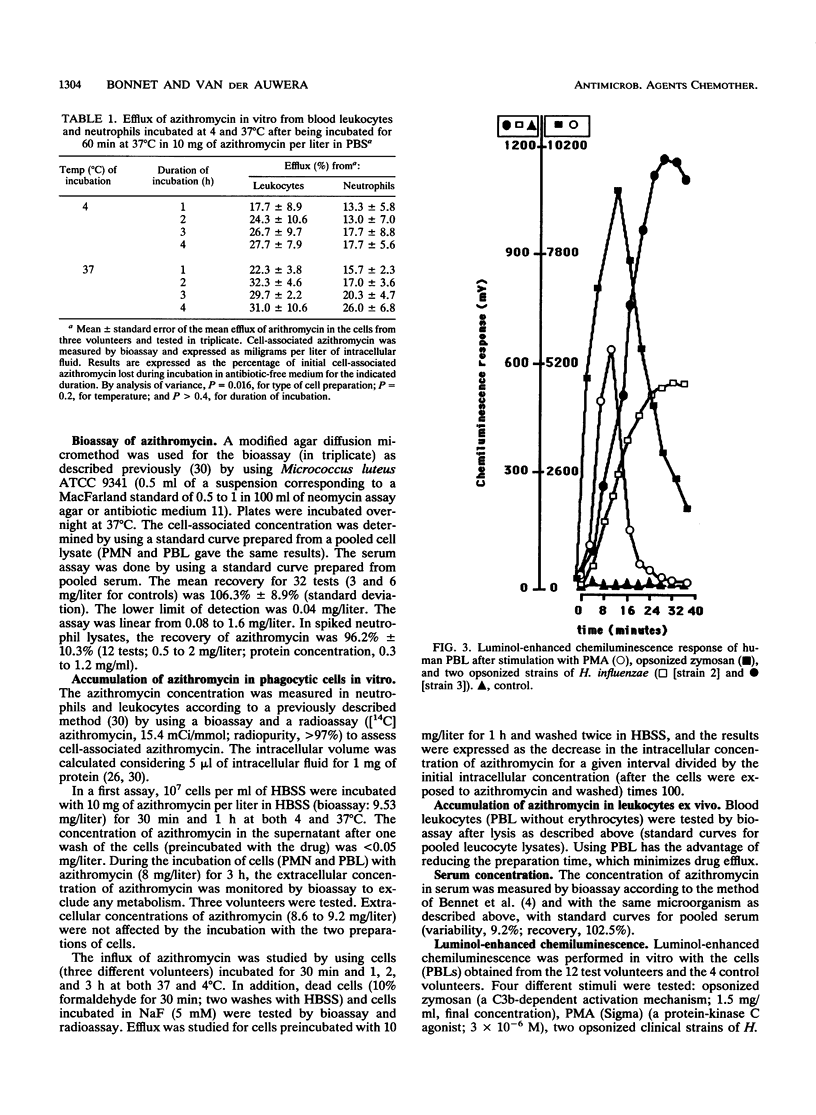
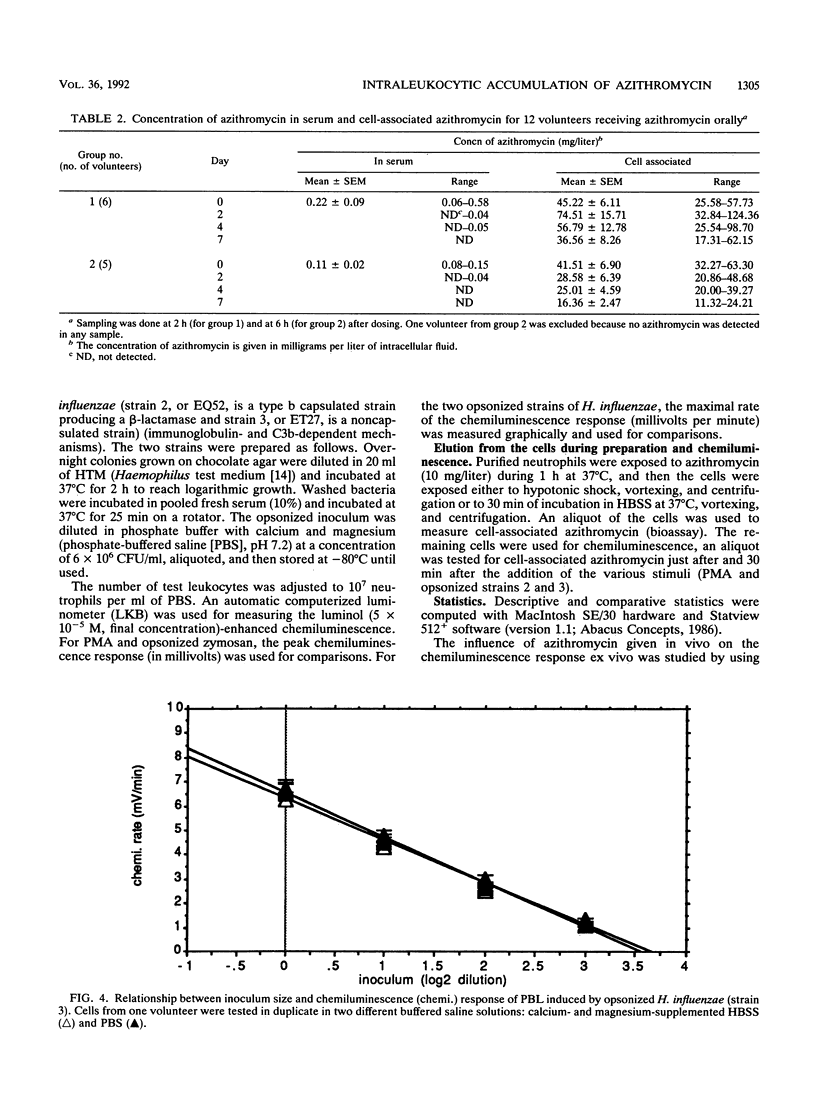
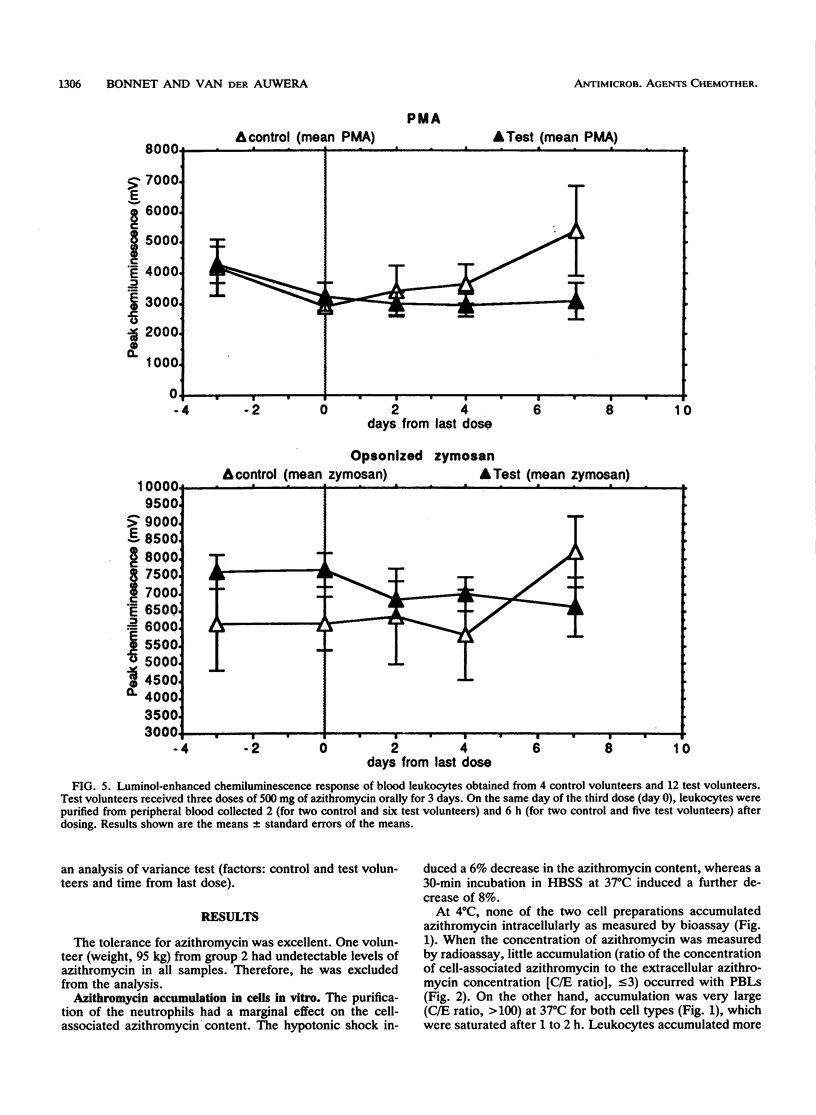
The accumulation of azithromycin in phagocytic cells was studied both in vitro by using a radiolabelled drug and a bioassay and in vivo for 12 volunteers receiving 1.5 g (total dose) orally within 3 days. In vitro, neutrophils and unfractionated blood leukocytes accumulated azithromycin up to 160-fold the extracellular concentration within 1 h at 37 degrees C but less than 3-fold at 4 degrees C. Dead cells accumulated up to 30-fold azithromycin, whereas NaF-treated cells accumulated up to 60-fold arithromycin. The mean efflux from preloaded cells was at most 31.0% +/- 10.6% (standard error of the mean) of the cell-associated concentration within 4 h of incubation at 37 degrees C in drug-free buffer. In vivo, the azithromycin concentration was 45.2 +/- 6.1 mg/liter of intracellular fluid at 2 h after the third dose and 36.6 +/- 8.3 mg/liter at 1 week thereafter. The corresponding concentrations in serum were 0.2 +/- 0.1 (2 h) and less than 0.05 (1 week). The luminol-enhanced chemiluminescence response induced by phorbol myristate acetate, opsonized zymosan, and two opsonized strains of Haemophilus influenzae (a type b capsulated strain and a noncapsulated strain) was also studied ex vivo by using the blood leukocytes from the 12 test volunteers and 4 control volunteers at 2 and 6 h after the third oral dose of azithromycin and at 2, 4, and 7 days thereafter. Azithromycin did not influence this response despite high levels of cellular accumulation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Joone G., van Rensburg C. E. An in vitro investigation of the intracellular bioactivity of amoxicillin, clindamycin, and erythromycin for Staphylococcus aureus. J Infect Dis. 1986 Mar;153(3):593–600. doi: 10.1093/infdis/153.3.593. [DOI] [PubMed] [Google Scholar]
- Anderson R., Joone G., van Rensburg C. E. An in-vitro evaluation of the cellular uptake and intraphagocytic bioactivity of clarithromycin (A-56268, TE-031), a new macrolide antimicrobial agent. J Antimicrob Chemother. 1988 Dec;22(6):923–933. doi: 10.1093/jac/22.6.923. [DOI] [PubMed] [Google Scholar]
- Anderson R., Van Rensburg C. E., Eftychis H., Jooné G., van Rensburg A. J. Further studies on erythromycin effects on cellular immune functions in vitro and in vivo. Enhancement of neutrophil motility by erythromycin combined with ascorbate or thiamine. J Antimicrob Chemother. 1982 Nov;10(5):409–417. doi: 10.1093/jac/10.5.409. [DOI] [PubMed] [Google Scholar]
- Bennett J. V., Brodie J. L., Benner E. J., Kirby W. M. Simplified, accurate method for antibiotic assay of clinical specimens. Appl Microbiol. 1966 Mar;14(2):170–177. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E., Inderlied C., Young L. S. Stimulation with cytokines enhances penetration of azithromycin into human macrophages. Antimicrob Agents Chemother. 1991 Dec;35(12):2625–2629. doi: 10.1128/aac.35.12.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M. B., Zenebergh A., Tulkens P. M. Cellular uptake and subcellular distribution of roxithromycin and erythromycin in phagocytic cells. J Antimicrob Chemother. 1987 Nov;20 (Suppl B):47–56. doi: 10.1093/jac/20.suppl_b.47. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., Long G. D., Shirley P. S., Bass D. A., Thomas M. J., Henderson F. W., Cohen M. S. Mechanism of the luminol-dependent chemiluminescence of human neutrophils. J Immunol. 1982 Oct;129(4):1589–1593. [PubMed] [Google Scholar]
- Fitzgeorge R. B., Featherstone A. S., Baskerville A. Efficacy of azithromycin in the treatment of guinea pigs infected with Legionella pneumophila by aerosol. J Antimicrob Chemother. 1990 Jan;25 (Suppl A):101–108. doi: 10.1093/jac/25.suppl_a.101. [DOI] [PubMed] [Google Scholar]
- Girard A. E., Girard D., English A. R., Gootz T. D., Cimochowski C. R., Faiella J. A., Haskell S. L., Retsema J. A. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother. 1987 Dec;31(12):1948–1954. doi: 10.1128/aac.31.12.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladue R. P., Bright G. M., Isaacson R. E., Newborg M. F. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob Agents Chemother. 1989 Mar;33(3):277–282. doi: 10.1128/aac.33.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand W. L., Hand D. L., King-Thompson N. L. Antibiotic inhibition of the respiratory burst response in human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1990 May;34(5):863–870. doi: 10.1128/aac.34.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N., Holman J. W. Entry of roxithromycin (RU 965), imipenem, cefotaxime, trimethoprim, and metronidazole into human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1987 Oct;31(10):1553–1557. doi: 10.1128/aac.31.10.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro M., Koga H., Kohno S., Hayashi T., Yamaguchi K., Hirota M. Penetration of macrolides into human polymorphonuclear leucocytes. J Antimicrob Chemother. 1989 Nov;24(5):719–729. doi: 10.1093/jac/24.5.719. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. H., Redding J. S., Maher L. A., Howell A. W. Improved medium for antimicrobial susceptibility testing of Haemophilus influenzae. J Clin Microbiol. 1987 Nov;25(11):2105–2113. doi: 10.1128/jcm.25.11.2105-2113.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga H. High-performance liquid chromatography measurement of antimicrobial concentrations in polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1987 Dec;31(12):1904–1908. doi: 10.1128/aac.31.12.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labro M. T., Amit N., Babin-Chevaye C., Hakim J. Synergy between RU 28965 (roxithromycin) and human neutrophils for bactericidal activity in vitro. Antimicrob Agents Chemother. 1986 Jul;30(1):137–142. doi: 10.1128/aac.30.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labro M. T., Bryskier A., Babin-Chevaye C., Hakim J. Interaction de la roxithromycine avec le polynucléaire neutrophile humain in vitro et ex vivo. Pathol Biol (Paris) 1988 Jun;36(5 Pt 2):711–714. [PubMed] [Google Scholar]
- Labro M. T., el Benna J., Babin-Chevaye C. Comparison of the in-vitro effect of several macrolides on the oxidative burst of human neutrophils. J Antimicrob Chemother. 1989 Oct;24(4):561–572. doi: 10.1093/jac/24.4.561. [DOI] [PubMed] [Google Scholar]
- Milatovic D. Intraphagocytic activity of erythromycin, roxithromycin and azithromycin. Eur J Clin Microbiol Infect Dis. 1990 Jan;9(1):33–35. doi: 10.1007/BF01969530. [DOI] [PubMed] [Google Scholar]
- Miller M. F., Martin J. R., Johnson P., Ulrich J. T., Rdzok E. J., Billing P. Erythromycin uptake and accumulation by human polymorphonuclear leukocytes and efficacy of erythromycin in killing ingested Legionella pneumophila. J Infect Dis. 1984 May;149(5):714–718. doi: 10.1093/infdis/149.5.714. [DOI] [PubMed] [Google Scholar]
- Miyachi Y., Yoshioka A., Imamura S., Niwa Y. Effect of antibiotics on the generation of reactive oxygen species. J Invest Dermatol. 1986 Apr;86(4):449–453. doi: 10.1111/1523-1747.ep12285793. [DOI] [PubMed] [Google Scholar]
- Prokesch R. C., Hand W. L. Antibiotic entry into human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1982 Mar;21(3):373–380. doi: 10.1128/aac.21.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghoebar M., Lindeyer E., Van den Berg W. B., Van Ginneken C. A. On the mechanisms of association of the macrolide antibiotic erythromycin with isolated human polymorphonuclear leucocytes. Biochem Pharmacol. 1988 Sep 1;37(17):3221–3227. doi: 10.1016/0006-2952(88)90631-4. [DOI] [PubMed] [Google Scholar]
- Steele R. W. Clinical applications of chemiluminescence of granulocytes. Rev Infect Dis. 1991 Sep-Oct;13(5):918–925. doi: 10.1093/clinids/13.5.918. [DOI] [PubMed] [Google Scholar]
- Steinberg T. H., Hand W. L. Effect of phagocyte membrane stimulation on antibiotic uptake and intracellular bactericidal activity. Antimicrob Agents Chemother. 1987 Apr;31(4):660–662. doi: 10.1128/aac.31.4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Brodie S. E., Cohn Z. A. Membrane flow during pinocytosis. A stereologic analysis. J Cell Biol. 1976 Mar;68(3):665–687. doi: 10.1083/jcb.68.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera P., Husson M., Frühling J. Influence of various antibiotics on phagocytosis of Staphylococcus aureus by human polymorphonuclear leucocytes. J Antimicrob Chemother. 1987 Sep;20(3):399–404. doi: 10.1093/jac/20.3.399. [DOI] [PubMed] [Google Scholar]
- Van der Auwera P., Matsumoto T., Husson M. Intraphagocytic penetration of antibiotics. J Antimicrob Chemother. 1988 Aug;22(2):185–192. doi: 10.1093/jac/22.2.185. [DOI] [PubMed] [Google Scholar]
- Van der Auwera P., Petrikkos G., Husson M., Klastersky J. Influence of various antibiotics on superoxide generation by normal human neutrophils. Arch Int Physiol Biochim. 1986 Dec;94(5):S23–S28. [PubMed] [Google Scholar]
- Walsh M., Kappus E. W., Quinn T. C. In vitro evaluation of CP-62,993, erythromycin, clindamycin, and tetracycline against Chlamydia trachomatis. Antimicrob Agents Chemother. 1987 May;31(5):811–812. doi: 10.1128/aac.31.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]



