Abstract
Interfibrillar mitochondria (IFM) of the heart in aged Fischer 344 rats show a biochemical defect which might be reflected in their morphology. We examined by high resolution scanning electron microscopy over 5,500 mitochondria to determine if a concomitant structural alteration existed. This methodology provides a means of examining mitochondrial cristae in three dimensions. Cristae of in situ subsarcolemmal mitochondria (SSM) and of IFM in both 6 and 24 month old Fischer rats are predominantly lamelliform. When isolated, these organelles, whether of SSM or IFM origin, display enhanced heterogeneity, but they have similar crista morphology irrespective of the age of the rat. Crista configuration does not play a major role in age-related cardiac mitochondrial defects.
Cardiac mitochondria exist in two functionally distinct populations. Subsarcolemmal mitochondria (SSM) are situated beneath the sarcolemma, whereas interfibrillar mitochondria (IFM) reside between the myofibrils (Palmer et al., 1977). Biochemically speaking, aging selectively alters IFM, with the protein yield and rate of oxidative phosphorylation being decreased, but SSM remain unaffected (Fannin et al., 1999). Despite these biochemical differences, transmission electron microscopy does not reveal any obvious morphologic changes in cardiac mitochondria associated with aging (Fannin et al., 1999).
Osmium extraction of tissues and pellets of isolated mitochondria combined with high resolution scanning electron microscopy (HRSEM) permits the examination of the interior of these organelles. Our use of osmium extraction-HRSEM allowed us to establish the three-dimensional structure of cristae in more than a thousand in situ and isolated cardiac mitochondria in or derived from young adult Sprague-Dawley rats (Riva et al., 2005). SSM contain largely lamelliform cristae; in contrast, cristae in IFM display mostly tubular morphology (Riva et al., 2005). This result provides a morphological underpinning for the previous biochemical studies of rat heart mitochondria that had shown functional differences between SSM and IFM.
We now turn our attention to the Fisher 344 rat, a long-established model for aging studies (Coleman et al., 1977). In this aging model, there is a disconnect between metabolic changes, which are highly significant, and the absence of micromorphological responses of mitochondria.
METHODS
Biochemistry
Male Fischer 344 (F344) rats aged 6 months (adult; N=6) and 24 months (elderly; N=5) that were maintained on NIH-31 Diet (7017) were obtained from the National Institute of Aging colony (Harlan Sprague Dawley, Inc. Indianapolis, IN). The Institutional Animal Care and Use Committees of the Louis Stokes Cleveland Department of Veterans Affairs Medical Center and of Case Western Reserve University School of Medicine approved the animal care and utilization protocol. SSM and IFM populations of cardiac mitochondria were isolated by a modification of the method of Palmer et al. (Palmer et al., 1977). SSM were isolated in Chappel-Perry buffer by homogenization of the myocardial mince with a Polytron tissue processor (Brinkman Instruments) at a rheostat setting of 6.0 for 2.5 seconds. IFM were isolated from polytron-skinned myofibers by incubation with 5 mg/g (wet weight) trypsin for 10 min at 4°C (Moghaddas et al., 2002; Chen et al., 2006).
Scanning Electron Microscopy
For morphological studies in the 6 and 24 month-old rats hearts from the above cited rats were used for in situ studies. Specimens of both in situ tissues and mitochondrial pellets were prepared for HRSEM by our previously published protocol (Riva et al., 1999; Riva et al., 2005). Fixed solid tissues were sectioned at 150 μm with a Sorvall TC2 tissue sectioner at room temperature. Mitochondrial pellets were frozen in liquid nitrogen, then shattered by a blow from a steel rod. The sections or pellet fragments were osmium-extracted (macerated) by immersion in 0.1% osmium for 44–48 hours. These samples were dehydrated in acetone, then critical point-dried with carbon dioxide. The sections or fragments were sputter-coated with platinum and examined in an FE Hitachi S4000 scanning electron microscope operating at 15–20 Kv.
Of the heart specimens, only myofibers that had been cut in transverse section were utilized in this study, so that the intracardiomyocyte position of the counted mitochondria was incontrovertible. Both SSM and IFM were identified—in order for an organelle to be considered an SSM, a portion of its outer surface had to abut the sarcolemma; to be considered an IFM, an organelle had to be some distance removed from the sarcolemma, with no obvious extension to the myocyte surface.
Statistical Analysis
Data are expressed as mean ± standard deviation. Differences between adult and aged hearts are compared by non-parametric analysis using the Mann-Whitney test of statistical significance. Within each subpopulation of mitochondria, the effect of age (6 or 24 mo.) on the distribution of crista morphology (lamelliform, mixed, tubular) is compared by a rectangular chi-square analysis (Matthews and Farewell, 1996). A p<0.05 is considered significant.
RESULTS
Biochemistry
Consistent with our previous results (Fannin et al., 1999), in IFM aging decreased the rate of state 3 respiration, the maximal ADP-stimulated rate of oxidative phosphorylation, and uncoupled respiration by 30–40% (data not shown). The rate of state 3 and uncoupled respiration was not significantly different in SSM from the elderly rat compared to the adult, whereas the maximal ADP-stimulated respiratory rate of oxidative phosphorylation was decreased in SSM from aged rats. In both age groups, oxidative phosphorylation was well-coupled in both populations of mitochondria.
Scanning electron microscopy
The morphology of mitochondrial cristae was evaluated in extracted specimens of myocardial tissue and was used as the basis for their classification as either lamelliform, mixed, or tubular. HRSEM analysis of in situ myocardium from the aged heart revealed that in terms of crista configuration SSM (Fig. 3; 535 counted, 86±7% lamelliform) in 24 month rats are similar to those in their 6 month counterparts (Fig. 2; 713 counted, 87±6%, p=NS); in other words, the percentage of organelles with lamelliform cristae remains the same (Fig. 6). In like fashion, the IFM in 24 month aged rats (Fig. 3; 847 counted, 84±10%) also morphologically closely resemble those present in the 6 month hearts (Fig. 2; 591 counted, 75±19%, p=NS); put another way, the IFM do not show morphological alterations in concert with the oxidative defect (Fig. 6).
Fig. 3.
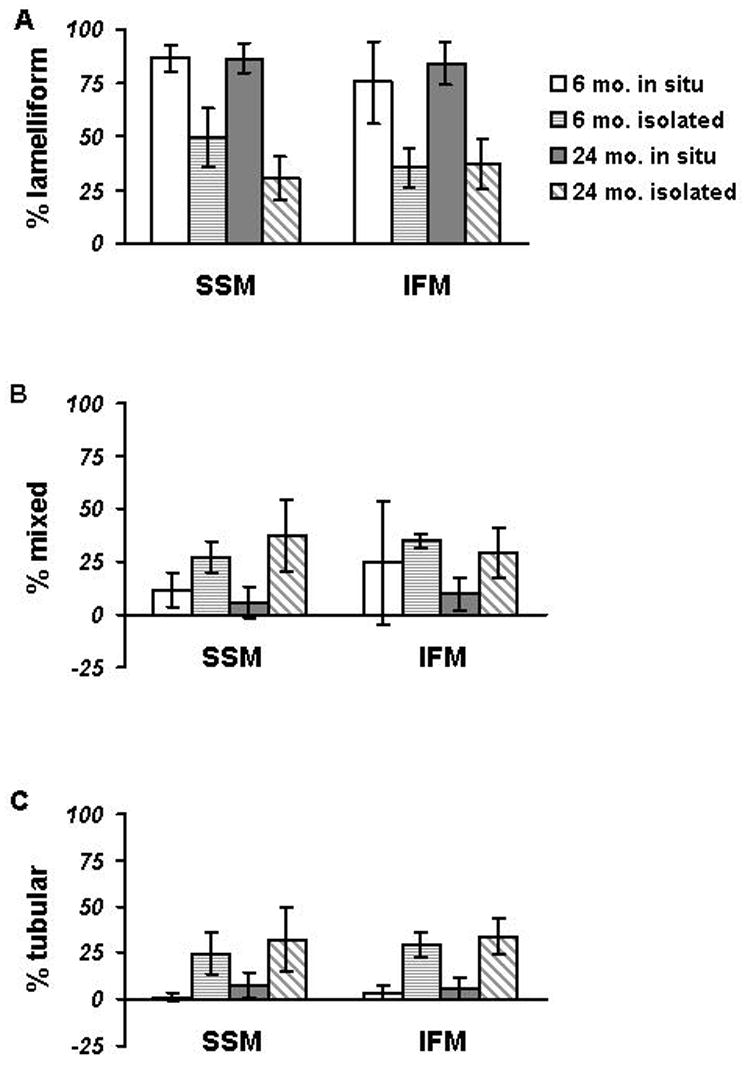
Histogram of cristal morphology in in situ mitochondria in heart from 6 and 24 month-old Fischer 344 rats and their isolated counterparts. In the hearts from 6 month-old rats, the percentages are based on 713 SSM and 591 IFM, whereas from 24 month-old rats, the percentages are based on 535 SSM and 847 IFM. In the mitochondria from 6 month-old rats, the percentages are based on 625 SSM and 818 IFM, whereas from 24 month-old rats the percentages are based on 699 SSM and 691 IFM.
A. The top panel shows the percentage of mitochondria with exclusively lamelliform cristae.
B. The middle panel shows data for mitochondria with mixed cristae.
C. The bottom panel shows the percentage of mitochondria with solely tubular cristae.
column - open: 6 mo. in situ
column - with horizontal lines: 6 mo. isolated
column - with gray fill: 24 mo. in situ
column - with diagonal lines: 24 mo. isolated
Fig. 2.
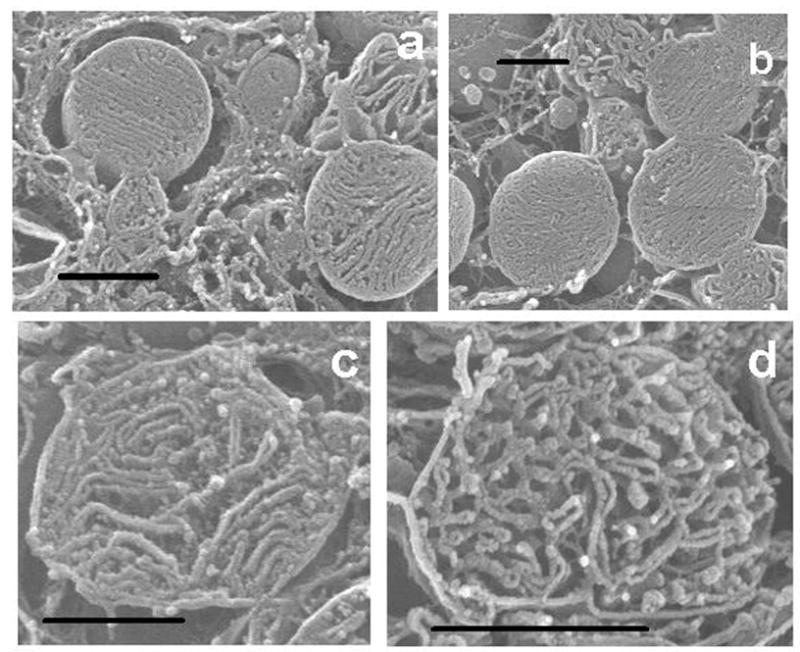
Fig. 2a. Isolated and osmium-extracted SSM from a six month-old rat. The cristae are almost exclusively lamelliform. The tangled ribbons in the background in this and subsequent micrographs are strands of agarose in which the mitochondrial pellets are embedded.
Fig. 2b. Isolated and osmium-extracted IFM from a six month-old rat. The cristae are predominantly lamelliform.
Fig. 2c. Isolated and osmium-extracted SSM from a 24 month-old rat. The cristae are a mixture of lamelliform and tubular.
Fig. 2d. Isolated and osmium-extracted IFM from a 24 month-old rat. The cristae are mainly anastomizing tubules intermixed with sparse lamelliform ones. Bar, 1 um.
Unlike their in situ counterparts in both 6 and 24 month old rats, wherein most SSM (Figs. 5 A, B) have lamelliform cristae (Fig. 8), isolated SSM (625 mitochondria counted of 6 month) have a broader range of crista morphology (Fig. 6). In the isolated SSM there is a decrease in the percentage of organelles with exclusively lamelliform cristae (49±14%) accompanied by an increase in mitochondria with mixed (27±7%) or tubular cristae (25±11%). The same situation prevails in SSM isolated from the hearts of 24 month old rats (Fig. 6). Like isolated SSM, isolated IFM (Fig. 4B) display the same range of crista morphological reorganization as do SSM. Again, no age-related effect is observed in the isolated IFM from 24 month rat hearts (Fig. 5B; Fig. 6). That is to say, of the 691 IFM counted, exclusively lamelliform accounted for 37±11%, tubular accounted for 29±12%, and mixed accounted for 34±10%.
DISCUSSION
Our present biochemical observations match the findings of our previous studies of cardiac mitochondria in the Fischer 344 rat aging model (Fannin et al., 1999). Our morphological study found that in the six month-old Fischer 344 rat SSM predominantly (87±6%) are of the lamelliform type; this percentage did not significantly change in the aged Fischer 344 rat (86±7%). In the adult Fischer 344 rat IFM have mainly lamelliform cristae (75±19%); again, this percentage of IFM remained unaltered in the aged Fischer 344 rat (84±10%).
Based on TEM examination of Fischer 344 rat heart, SSM and IFM are structurally identical (Palmer et al., 1977; Palmer et al., 1985) and are unaltered by age (Fannin et al., 1999). HRSEM combined with osmium extraction provides a means for examining the interior of mitochondria in three dimensions. By extracting all soluble components of these organelles, their constituent membranes are rendered in stark relief. With this technique, it is possible to examine the internal organization, i.e., crista configuration, of vast numbers of organelles with relative ease. As a baseline for the current study, we examined by HRSEM the cardiac mitochondria of adult Sprague-Dawley rats (Riva et al., 2005). In this rat strain, 77% of the in situ SSM had lamelliform cristae exclusively, whereas of the IFM 55% were tubular and 24% mixed. Counts of isolated SSM revealed that these organelles resembled their in situ counterparts, with 75% having lamelliform cristae. Isolated IFM are relatively plastic in that only 20% retained tubular cristae and 58% were mixed.
Comparison of in situ mitochondria in the two strains of adult rats we have studied, i.e., Sprague-Dawley and Fischer 344, reveals that SSM have predominantly lamelliform cristae. In contrast, IFM in Sprague-Dawley rats are predominantly tubular, whereas their counterparts in Fischer rats are mainly lamelliform. Clearly there is a strain difference in cardiac mitochondrial crista configuration whether the organelles are in situ or are isolated; the basis for this difference is unclear.
A possible explanation for this strain difference is alteration in one rat strain of crista membrane structural components such as proteins and phospholipids, which could influence the final shape of the cristae. This plasticity of cristae has been noted under various physiological conditions. For example, kidney mitochondria in active frogs have transverse cristae whereas during hibernation, when the mitochondrial content of cytochrome oxidase is much reduced, the cristae become reoriented in a longitudinal direction (Karnovsky, 1963).
The lack of morphologic change with aging in IFM provides valuable information. It should be noted that our previous transmission electron microscopy study did not reveal any relevant ultrastructural alterations (Fannin et al., 1999), a finding echoed by our current HRSEM study. Cardiolipin, a key component of the mitochondrial inner membrane (Hoch, 1992) that contributes to dynamic structural alterations of inner membrane morphology (Hoch, 1992; McMillin and Dowhan, 2002), is unaltered with aging in IFM (Moghaddas et al., 2002). This is consistent with our HRSEM observations of unaltered crista morphology. In addition, cardiolipin is a critical cofactor of cytochrome oxidase that is located in the cristal components of the inner mitochondrial membrane (Scorrano et al., 2002). Aging decreases cytochrome oxidase activity in IFM despite a preserved cardiolipin content (Fannin et al., 1999; Lesnefsky et al., 2002; Moghaddas et al., 2002). This biochemical defect does not influence crista morphology with aging.
In sum, our HRSEM observations of in situ as well as isolated populations of mitochondria did not disclose age-related changes in IFM, which is the population exhibiting aging-induced oxidative defects, nor in SSM. It appears that aging-related biochemical defects do not affect the configuration of the mitochondrial cristae, even though this is where the electron transport chain complexes reside that are compromised with age. In the Sprague-Dawley rat strain, major differences between SSM and IFM were clearly evident by HRSEM (Riva et al., 2005).
The profound biochemical changes that we have described in IFM are not paralleled by similar age-related alterations in crista morphology. It is obvious in the aged Fischer 344 rat that the biochemical performance of mitochondria is not in lock-step with morphology. It can be concluded that in this system oxidative metabolism and the architecture of mitochondrial inner membrane are governed by independent mechanisms.
Fig. 1.
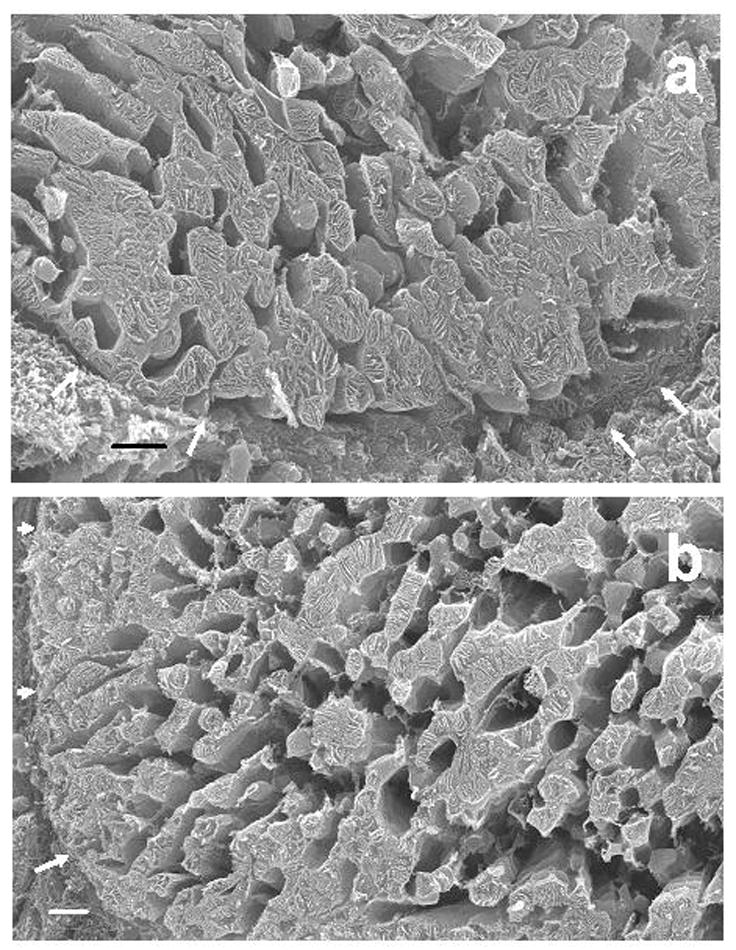
Fig. 1a. HRSEM micrograph of a transversely-sectioned cardiomyocyte from a six month-old rat. The internal structure of individual mitochondria is readily apparent. There are no obvious structural differences between those organelles (SSM) that abut the sarcolemma (arrows) and those (IFM) that are some distance removed from the latter. The lacunae between the mitochondria are sites that formerly were occupied by myofibrils that were extracted by long-term exposure to osmium tetroxide.
Fig. 1b. HRSEM micrograph of a transversely-sectioned cardiomyocyte from a 24 month-old rat. There are no essential differences in mitochondrial structure from that in the six month-old rat. The sarcolemma is indicated by arrows. Bar, 2 um.
Acknowledgments
The work was supported in part by NIH grant P01 AG15885, the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, and a grant from the Fondazione Banco di Sardegna. The assistance with statistical analysis by George Kypriotakis and with graphics by Ms. Rachel Floyd is appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chen Q, Hoppel CL, Lesnefsky EJ. Blockade of electron transport before cardiac ischemia with the reversible inhibitor amobarbital protects rat heart mitochondria. J Pharmacol Exp Ther. 2006;316:200–207. doi: 10.1124/jpet.105.091702. [DOI] [PubMed] [Google Scholar]
- Coleman GL, Barthold W, Osbaldiston GW, Foster SJ, Jonas AM. Pathological changes during aging in barrier-reared Fischer 344 male rats. J Gerontol. 1977;32:258–278. doi: 10.1093/geronj/32.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO, Hoppel CL. Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch Biochem Biophys. 1999;372:399–407. doi: 10.1006/abbi.1999.1508. [DOI] [PubMed] [Google Scholar]
- Hoch FL. Cardiolipins and biomembrane function. Biochim Biophys Acta. 1992;1113:71–133. doi: 10.1016/0304-4157(92)90035-9. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ. The fine structure of mitochondria in the frog nephron correlated with cytochrome oxidase activity. Exp Mol Pathol. 1963;40:347–366. doi: 10.1016/0014-4800(63)90016-9. [DOI] [PubMed] [Google Scholar]
- Krahenbuhl S, Chang M, Brass EP, Hoppel CL. Decreased activities of ubiquinol:ferricytochrome c oxidoreductase (complex III) and ferrocytochrome c:oxygen oxidoreductase (complex IV) in liver mitochondria from rats with hydroxycobalamin[c-lactam]-induced methylmalonic aciduria. J Biol Chem. 1991;266:20998–21003. [PubMed] [Google Scholar]
- Lesnefsky EJ, Tandler B, Moghaddas S, Hassan MO, Hoppel C. Mitochondrial electron transport and aging in the heart. In: Mattson MP, editor. Mechanisms of Cardiovascular Aging. Elsevier; San Diego: 2002. pp. 201–232. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Matthews DE, Farewell VT. In: Using and Understanding Medical Statistics. Karger, editor. Basel; Switzerland: 1996. [Google Scholar]
- McMillin JB, Dowhan W. Cardiolipin and apoptosis. Biochim Biophys Acta. 2002;1585:97–107. doi: 10.1016/s1388-1981(02)00329-3. [DOI] [PubMed] [Google Scholar]
- Moghaddas S, Stoll MS, Minkler PE, Salomon RG, Hoppel CL, Lesnefsky EJ. Preservation of cardiolipin content during aging in rat heart interfibrillar mitochondria. J Gerontol A Biol Sci Med Sci. 2002;57:B22–28. doi: 10.1093/gerona/57.1.b22. [DOI] [PubMed] [Google Scholar]
- Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977;252:8731–8739. [PubMed] [Google Scholar]
- Palmer JW, Tandler B, Hoppel CL. Biochemical differences between subsarcolemmal and interfibrillar mitochondria from rat cardiac muscle: effects of procedural manipulations. Arch Biochem Biophys. 1985;236:691–702. doi: 10.1016/0003-9861(85)90675-7. [DOI] [PubMed] [Google Scholar]
- Riva A, Faa G, Loffredo F, Piludu M, F TR. An improved OsO4 maceration method for the visualization of internal structures and surfaces in human bioptic specimens by high resolution scanning electron microscopy. Scanning Microsc. 1999;13:111–122. [Google Scholar]
- Riva A, Tandler B, Loffredo F, Vazquez E, Hoppel C. Structural differences in two biochemically defined populations of cardiac mitochondria. Am J Physiol Heart Circ Physiol. 2005;289:H868–872. doi: 10.1152/ajpheart.00866.2004. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, Korsmeyer SJ. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]


