Abstract
Red clover (Trifolium pratense L., Fabaceae) dietary supplements are currently used to treat menopausal symptoms because of their high content of the mildly estrogenic isoflavones daidzein, genistein, formononetin and biochanin A. These compounds are estrogenic in vitro and in vivo, but little information exists on the best time to harvest red clover fields to maximize content of the isoflavones and thus make an optimal product. Samples of cultivated red clover aboveground parts and flower heads were collected in parallel over one growing season in northeastern Illinois. Generally, autohydrolytic extracts of aboveground parts contained more isoflavones and had more estrogenic activity in Ishikawa endometrial cells, compared with extracts of flower heads. Daidzein and genistein content peaked around June to July, while formononetin and biochanin A content peaked in early September. Flower head and total aboveground parts extracts exhibited differential estrogenic activity in an Ishikawa (endometrial) cell-based alkaline phosphatase (AP) induction assay, whereas nondifferential activity was observed for most extracts tested in an MCF-7 (breast) cell proliferation assay when tested at the same final concentrations. Ishikawa assay results could be mapped onto the extracts’ content of individual isoflavones, but MCF-7 results did not show such a pattern. These results suggest that significant metabolism of isoflavones may occur in MCF-7 cells, but not in Ishikawa cells, and therefore caution is advised in the choice of bioassay used for the biological standardization of botanical dietary supplements.
Keywords: botanical dietary supplement, estrogenic activity, HPLC, Ishikawa bioassay, isoflavone content, MCF-7 cell proliferation, red clover, seasonal variation, Trifolium pratense
INTRODUCTION
Red clover (Trifolium pratense L., Fabaceae) is a perennial fabaceous plant native to the Mediterranean that is found widespread throughout the entire United States (1). It is mainly cultivated to feed livestock, but has also been used medicinally to treat whooping cough (2), asthma, eczema, eye diseases, and external cancers (3). Herbalists consider red clover an antispasmodic (4), expectorant (5), alterative (6), and sedative, and evidence exists that the Iroquois women used it during the “change of life” (7). Recent use of red clover revolves around its high content of the estrogenic isoflavones daidzein (1), genistein (2), formononetin (3) and biochanin A (4) (see Figure 1), and this plant is hypothesized to be of potential use in menopause as a natural form of hormone replacement therapy.
Figure 1.
Chemical structures of the four isoflavones measured in the present study of seasonal isoflavone variation in red clover collections.
Initial interest in red clover’s isoflavone content was spurred by research into the cause of sheep infertility in Australia during the 1930s and 1940s (8, 9). Female sheep grazing longer than one month on either subterranean (T. subterraneum L.) or red clover experienced difficulty conceiving and giving birth. Isoflavones, particularly formononetin, were found to be the estrogenic culprits responsible for this “clover disease.” Further studies revealed that other Trifolium species also contained especially high concentrations of isoflavones: T. alpestre L. (10), T. baccarini Chiov., T. tembense Fresen., T. globosum L., T. israeliticum D. Zohary & Katzn., T. pilulare Boiss. and T. lappaceum L. (11). Biological experiments indicated that red clover feed increased uterine weight in immature (12, 13) and ovariectomized (14) mice, and that the leaves were more potent than the flower heads (15). However, the relative estrogenic potency of red clover plant organs remains unclear. At least two studies have reported higher isoflavone content in red clover flower heads compared with leaves (10, 16), and another study compared isoflavone content in leaves to that of stems and petioles, but did not examine flower heads (17). Thus, ambiguity exists over which part(s) of red clover should be used to produce high-potency estrogenic isoflavone supplements.
Contradictory results also exist with regards to studies on the seasonal variation of isoflavones in red clover. This is partly due to fluctuations in chemical content according to geographic location, differing photoperiods of sunlight, weather, soil fertility, and partly because of differences in the sensitivity and specificity of older analytical chemical methods used to quantify isoflavones (10). Early methods used to quantitate isoflavones in red clover were based on TLC or paper chromatography, using densitometric or fluorometric detection (11, 16–19). These methods suffered from inconsistencies caused by the difficulty of achieving a clean chromatographic separation of formononetin from biochanin A using TLC, as well as the unstable fluorescence of formononetin after exposure to ammonia-containing reagents (17). It was also discovered that higher yields of isoflavone aglycones, compared with their glycosides, are achieved when the plant material is crushed before extraction (11, 19), due to enzymatic autohydrolytic release of aglycones from the stored glycosides within the plant, and this is another likely source of variation when quantitating isoflavones in red clover plant samples. A few studies have examined seasonal variation of isoflavones using HPLC-based detection methods (10, 18), which are considered to be more specific for the particular isoflavones under investigation because of their better chromatographic separation.
In the present study on the seasonal variation of daidzein, genistein, formononetin and biochanin A in red clover, we used an HPLC method to quantitate the individual isoflavones of autohydrolytic extracts prepared from red clover aboveground parts and flower heads collected in parallel over one growing season (June through October) in the U.S.A. (Illinois). As far as we are aware, this is the first study of the seasonal variation of red clover isoflavones carried out in the United States. In addition to chemical evaluation we also tested the extracts for estrogenic (agonistic) activity using the Ishikawa endometrial cell-based alkaline phosphatase (AP) induction and MCF-7 breast cell proliferation assays.
MATERIALS AND METHODS
Chemicals and Reagents
All chemicals and reagents were purchased from Fisher (Hanover Park, IL) or Sigma (St. Louis, MO), unless otherwise indicated. XTT [111072-31-2] was from the NCI-Frederick Repository (Frederick, MD). The standards daidzein (99% purity, estimated from NMR), genistein (99% purity, estimated from NMR), formononetin (98% purity, estimated from NMR) and biochanin A (95% purity, estimated from NMR) were from Indofine Chemical Co. (Somerville, NJ). Media for cell culture were purchased from Life Technologies (Grand Island, NY) and Invitrogen (Carlsbad, CA), and fetal bovine serum (FBS) was from Atlanta Biologicals (Norcross, GA), Mediatech, Inc. (Herndon, VA), and Hyclone (Logan, UT).
Plant Material
Trifolium pratense var. Mammoth was cultivated at the University of Illinois Pharmacognosy Field Station (Downer’s Grove, IL) in 2001. Seed was purchased from Johnny’s Selected Seeds (1 Foss Hill Rd., RR 1 Box 2580, Albion, Maine, 04910-9731) in the summer of 1999. Parallel collections of total aboveground parts (containing leaves, stems, petioles, and flower heads) and just flower heads were made during the hours of 0900-1500 approximately every two weeks over the course of one growing season (June-October 2001), for a total of eight collections. Exact dates of collections were: 6/26, 7/10, 7/24, 8/22, 9/04, 9/18, 10/03, and 10/18. Flowers were not present in similar quantities compared to total aboveground parts on every collection day. Samples were dried for at least ten days at ambient temperature in a shaded greenhouse that excluded 60–70% of sun, to prevent mold formation, and then were coarsely milled.
Extraction of Plant Material
Exactly 5.0 g of dried and milled flower heads or aboveground parts were weighed in a 500 mL Erlenmeyer flask and covered completely with 140 mL of doubly-deionized water (bioassay grade), covered, and left to stand overnight in order to liberate compartmentalized plant β-glucosidases and automatically hydrolyze the isoflavone glycosides to the aglycone forms (19). The next day, extracts were filtered using a Büchner funnel with #1 Whatman paper and filtrates were collected. A second overnight water extraction was done using 100 mL of doubly-deionized water. The spent plant material was extracted yet again overnight with 200 mL of 100% non-denatured ethanol and then a fourth time with 70% aqueous non-denatured ethanol. All four filtrates from each sample were combined as one extract and concentrated to dryness in vacuo. The dried extracts were weighed and then transferred to amber vials for storage at −20 °C.
Preparation of Extracts for HPLC and Bioassay
For HPLC analysis, 200–250 mg of each dry extract was accurately weighed into a 10 mL volumetric flask. Methanol (100%) was added to cover the solid (~5 mL), the flask was stoppered and the mixture was sonicated for at least 10 minutes, and then allowed to stand at room temperature overnight or until time of analysis. On the day of HPLC analysis, methanol was quantitatively added to the flask to a total volume of exactly 10 mL, the mixture was sonicated, and then was allowed to stand at room temperature for at least one hour to allow particulate matter to settle. One mL of solution was filtered through a 13 mm 0.2 μm nylon syringe filter and discarded. Then more extract was filtered using the equilibrated filter and collected for the HPLC analysis.
Due to the low solubility of the aqueous alcoholic red clover extracts in 100% dimethylsulfoxide (DMSO), extracts were prepared in 50% aqueous DMSO at an initial (prior to biological testing) concentration of 40 mg/mL. One part water that was half the final total volume needed to make the solution 40 mg/mL was added to a weighed amount of extract and the mixture was sonicated and warmed intermittently for ≤ 30 minutes to ensure solubilization of components. Then one part DMSO was added and the suspension was again sonicated and warmed intermittently for ≤ 30 minutes. Samples were transferred into 2 mL microcentrifuge tubes and centrifuged for 15–20 minutes at 15,000 × g. The supernatant was carefully removed with a micropipettor and transferred to a fresh microcentrifuge tube. The process was repeated until a clear solution without suspended particles was achieved (2–3 times). All solutions were stored at −20 °C until testing.
HPLC Analysis of Extracts for Isoflavone Content
Methanol solutions prepared as described under Preparation of Extracts for HPLC and Bioassay were transferred into 1.8 mL glass robovials having 150 μL glass inserts and rubber/silicone septa screw caps. Injections of each sample (10 μL) were analyzed in triplicate using a Beckman ODS μBondapak 4.2 × 250 mm (I.D. × length) column attached to an Agilent 1100 analytical HPLC with diode array detector set to detect absorbance at 254 nm. A 60-minute method was employed, with flow rate = 1.5 mL/min and gradient scheme as follows: 100% A/0% B to 60% A/40% B from 0–20 min, hold at 60% A/40% B from 20–22 min, 60% A/40% B to 10% A/90% B from 22–60 min, followed by a 10 min column wash of 100% B and then re-equilibration to 100% A for 14 min before the next injection, where A = 85% doubly-deionized water/15% HPLC-grade acetonitrile/0.1% HPLC-grade glacial acetic acid (v/v/v) and B = 50% doubly-deionized water/50% HPLC-grade acetonitrile/0.1% HPLC-grade glacial acetic acid (v/v/v). Standard curves were generated for the four isoflavones. Injections of a mixture of the 4 isoflavones were performed after every nine sample injections to compensate for any drifting of retention times and to verify the identity of the four isoflavones in the extract samples. Areas under the curve for each of the four isoflavone peaks present in the extracts were determined using ChemStation software, and these areas were used to calculate the percent weight of isoflavones present in the samples, based on the standard curve linear regression equation and amount (μg) injected onto the column. When areas of peaks for experimental samples fell below the range of the standard curve for a compound, samples were declared to have zero content of that isoflavone. Relative standard deviations (RSDs) for area values from triplicate injections were calculated as [(mean – standard deviation)/mean] × 100, and sample RSDs had to be < 5% to be considered valid data. Concentrations of each of the four isoflavones were calculated as a weight percent of the extract, and statistical analyses were done using these results. Results are presented in Figure 2. Table 1 gives the isoflavone percent ranges based on dry weight of the crude dry plant material.
Figure 2.
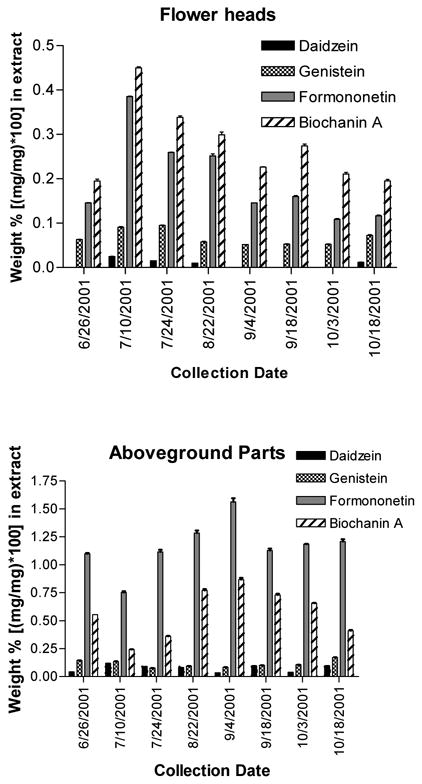
Isoflavone content of extracts of cultivated red clover aboveground parts and flower heads, expressed as weight percent of isoflavone found per extract [(mg isoflavone/mg extract) × 100]. Results indicate average ± standard deviation for triplicate analyses.
Table 1.
Calculated isoflavone content of red clover aboveground parts and flower heads, expressed as percent found per dry weight of plant [(mg isoflavone/mg dry plant) × 100].
| Weight percent | ||||
|---|---|---|---|---|
| Daidzein | Genistein | Formononetin | Biochanin A | |
| Aboveground parts | 0.013–0.033% | 0.014–0.068% | 0.21–0.59% | 0.07–0.33% |
| Flower heads | 0.0025–0.0079% | 0.018–0.019% | 0.047–0.12% | 0.070–0.14% |
| Limit of quantitation [ng/mL] | 50 | 125 | 130 | 540 |
Cell Culture Conditions
The Ishikawa cell line was provided by Dr. R. B. Hochberg (Yale University, New Haven, CT). Ishikawa cells were maintained in Dulbecco’s Modified Eagle medium (DMEM/F12) containing 1% sodium pyruvate (v/v), 1% non-essential amino acids (NEAA) (v/v), and 1% GlutaMAX™-1 (v/v) (Invitrogen, Carlsbad, CA), 0.05% insulin (v/v), and 10% heat-inactivated fetal bovine serum (FBS) (v/v). One day prior to treating the cells, the medium was replaced with phenol red-free DMEM/F12 medium containing the same supplements and charcoal/dextran-stripped FBS. The MCF-7 cell line was from the NCI-Frederick Repository (Frederick, MD) and cells were maintained in RPMI-1640 medium containing L-glutamine plus phenol red, supplemented with 10% (v/v) normal FBS. At least two days prior to seeding 96-well plates for the assay, MCF-7 cells were passaged into flasks using phenol red-free RPMI-1640 medium containing 10% dextran-coated charcoal-stripped FBS. Incubations were done in a 5% CO2 atmosphere at 37°C with saturated humidity.
Induction of Alkaline Phosphatase (AP) with Cultured Ishikawa Cells
Extracts were analyzed at a final concentration of 20 μg/mL for estrogenic (agonistic) activity using the Ishikawa cell AP induction assay described previously (20–21), except that the phosphatase substrate (p-nitrophenylphosphate) solution used was 1 mg/mL in concentration. Each sample was analyzed in triplicate, and results are reported as the mean ± standard deviation (see Figure 3). Concurrent evaluation of cytotoxicity was carried out, as previously described (20–21), but results are not reported because no sample had greater than 15% cytotoxicity.
Figure 3.
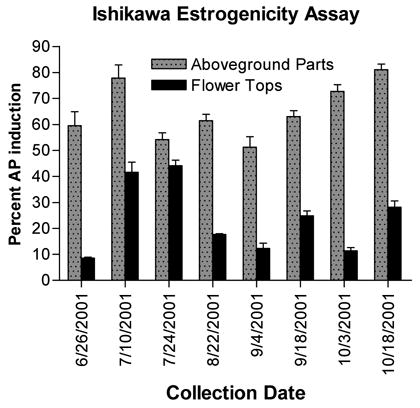
Estrogenic activity of the red clover extracts, measured as percent alkaline phosphatase induction in Ishikawa endometrial cells relative to a solvent control. Estradiol was included as a positive control (data not shown). Results shown are the average ± standard deviation for triplicate analyses.
Measuring MCF-7 Cell Proliferation
Extracts were tested at a final concentration of 20 μg/mL for cell proliferation activity using an XTT-based growth measurement assay optimized for the background amount of DMSO present in the extracts. After priming cells for at least two days in phenol red-free “clear” medium containing charcoal-stripped FBS, cells were trypsinized and plated, using the same medium, into 96-well flat-bottomed clear plastic plates at a cell density of 15 × 104 cells/mL, at 140 μl/well. Cells were allowed to rest overnight. The next day the red clover extracts, solvent blanks (negative control), 17β-estradiol (positive control), and tamoxifen citrate (negative and cytotoxicity controls) were diluted into clear medium at a ratio of 1.0:13.3 (v:v). Then 10 μl of each diluted extract or control was added to four replicate wells in the plate (1:15 dilution). Final concentrations were: 20 μg/mL extracts, 0.25% DMSO solvent blank, 1 μM estradiol, 10 and 30 μM tamoxifen citrate, respectively. Positive and negative controls contained the same final concentration of DMSO as did the extracts. Cells were incubated with extracts and the controls for three days, and then 50 μl of XTT solution was added to each well. XTT solution was freshly made and contained 1 mg/mL XTT and 4% (v/v) of a 0.158 mg/mL phenazine methosulfate (PMS; in Dulbecco’s phosphate-buffered saline) solution dissolved into phenol red-free RPMI-1640 medium containing L-glutamine but no FBS. Plates were incubated at 37°C in 5% CO2/saturated humidity for ~4 h and then absorbance at 450 nm (0.1 s/well) was read for each well using a Wallac 1420 workstation (PerkinElmer, Wellesley, MA). Cell blank controls were optimized to a final reading of ~1.5 absorbance units. Percent growth induction was calculated as a percentage of the average response of the estradiol control samples: [(absorbancesample – avg absorbancemedium)/(avg absorbanceestradiol – avg absorbance medium)] × 100, where 100% activity corresponds to the equivalent proliferation activity of 1 μM estradiol (see Figure 4). Results reported are the mean ± std deviation of four replicate determinations from a representative assay.
Figure 4.
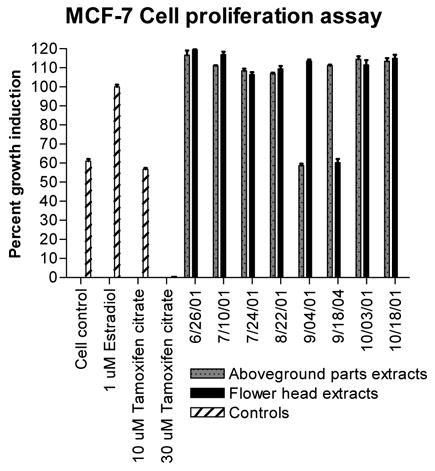
Induction of estrogen receptor-positive MCF-7 breast cell proliferation, a general measure of estrogenic activity, by the red clover extracts. XTT absorbance data were corrected for the background absorbance of the medium, and results are expressed relative to that of a 1 μM estradiol positive control. Results shown are the average ± standard deviation for quadruplicate analyses.
RESULTS AND DISCUSSION
In our HPLC system, daidzein, genistein, formononetin and biochanin A eluted within the following retention time ranges: 18.5–18.9, 27.6–27.9, 35.1–35.4, and 50.5–50.6 min, respectively. R2 values for the least-squares regression equations fitted to the standard curves were as follows: daidzein (1) (0.9996), genistein (2) (0.9992), formononetin (3) (0.9997), and biochanin A (4) (0.9979). Limits of quantitation were determined previously and were: 1 (50 ng/mL), 2 (125 ng/mL), 3 (130 ng/mL), 4 (540 ng/mL). None of the RSDs of samples exceeded 5%, indicating adequate injection reproducibility. Table 1 gives the ranges of weight percents observed for each isoflavone detected in the extracts of red clover aboveground parts and flower heads. Although we did not specifically measure content of isoflavone β-glucosides (daidzin, genistin, ononin, sissotrin, for 1 through 4, respectively), the region of the chromatogram where these compounds normally elute generally showed low levels or absence of these glucosides in the extracts, indicating mainly aglycone isoflavone content.
We found that the isoflavone content of extracts of red clover total aboveground parts were generally much higher than that of extracts of flower heads, and this was true for all four isoflavones investigated (see Figure 2). A general linear model with interaction was developed to analyze isoflavone content according to collection time and plant part. The model was utilized to analyze the percent (w/w) content in the extracts of each of the four compounds. Following the general fitting, a post hoc comparison was performed to examine the isoflavone content differences between aboveground parts and flower heads at each time point (extract). Tests were considered significant at p < 0.05, and all tests were two-sided. Results showed that 1, 2, 3, and 4 were, at nearly all instances, significantly higher in extracts of aboveground parts compared with extracts of flower heads, except that the content of 4 on 7/10/01 and the content of 2 on 7/24/01 was higher in flower heads than aboveground parts. On these dates, there were statistically significant differences in isoflavone content between the aboveground parts and flower heads. Our results demonstrate that total aboveground parts of red clover generally contain more isoflavones than flower heads alone, and would, therefore, be the optimal plant part to use in making isoflavone botanical dietary supplements.
For aboveground parts extracts, analysis of variance (ANOVA) was used to analyze individual isoflavone content within each of the four groups, followed by post hoc Bonferroni multiple comparison tests. Results indicated that 1 content on 7/10/01, 2 content on 6/26/01 and 7/10/01, 3 content on 9/04/01, and 4 content on 9/04/01 differed significantly (p < 0.05) from all other time points (α = 0.05) shown in Figure 2 for each isoflavone. Therefore, 1 and 2 levels in red clover aboveground parts appear to peak around July, while 3 and 4 levels peak in early September. These results present a quandary, as there are two potential harvesting dates. Compounds 3 and 4 by far make up the significant bulk weight of isoflavones in red clover extracts, compared with 1 and 2, which are usually only present in very low amounts. Additionally, in vivo, 3 and 4 are demethylated to form 1 and 2, respectively (22). Therefore, based solely on chemical content, we conclude that the best harvesting time for red clover aboveground parts under our growing conditions is early September. In Illinois, red clover generally flowers from late May through September. Varieties of red clover grown in other geographic locations may well present a different seasonal variation of isoflavone content.
An interesting point to note is that from 9/04/01 to 10/08/01, biochanin A levels appear to decrease, while there is a concomitant increase in genistein content in the aboveground part extracts. Formononetin decreases stepwise in flower head extracts during this period, but daidzein levels are less consistent. These trends suggest that there may be bioconversion of biochanin A (and possibly formononetin) into genistein (and daidzein) in the plant during the latter part of the growing season, but more studies are needed to confirm this hypothesis. Figure 5 depicts the average temperature and rainfall for the months when red clover collections were made. During the study period, the maximum average monthly temperature occurred in July, which correlates with the maximum isoflavone content found in the red clover flower head extracts. Alternatively, maximum rainfall occurred in September, which is when the aboveground part extracts contained the highest amount of isoflavones. These results suggest that temperature and precipitation affect the production and/or methylated storage forms of isoflavones in red clover aerial parts and flower heads. If supported by future studies, this correlation would allow medicinal herb farms to easily track the weather affecting red clover crops and determine optimal harvest times for highest isoflavone content, even for any lack of sophisticated analytical HPLC equipment. One might harvest red clover flower heads only in July, and then harvest the total aboveground parts in September, for use in differently marketed “medicinally active” herbal products.
Figure 5.
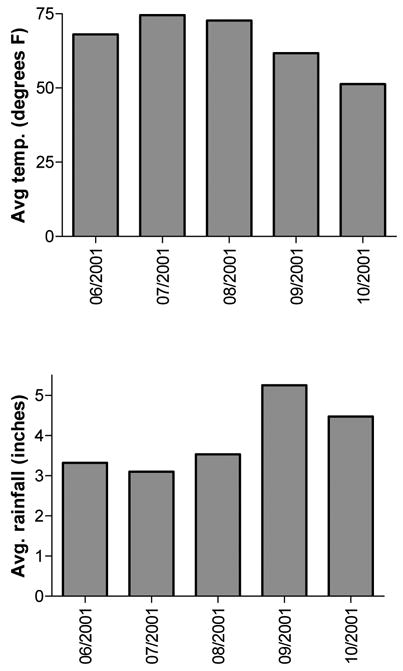
Average monthly temperature, in degrees Fahrenheit, and average monthly rainfall, in inches, during the course of the study. Data courtesy of USDA, National Agricultural Statistics Service.
Another general linear model with main effects (collecting time, replicate biological tests, and plant part) and both two-way and three-way interactions was developed to analyze the Ishikawa estrogenic activity of the red clover extracts. After fitting the model, a post hoc comparison between activities of aboveground parts versus flower heads was performed at each time point. All tests were two-sided and were considered significant if p < 0.05. It was quite obvious that estrogenic activities of the aboveground parts extracts were significantly higher than the activities of blossom extracts for every collection period. Estrogenic activity, as measured with the Ishikawa AP induction bioassay, correlates well with the maximum 2 and 1 content of the red clover aboveground parts extracts, as the highest activity is noted in extracts from plant material collected on 7/10/01 and 10/18/01 (see Figure 3). However, estrogenic activity is lower on the collection dates when 3 and 4 are highest. This observation is probably due to the fact that 1 and 2 are more estrogenic in Ishikawa cells, compared with 3 and 4, and because Ishikawa cells cannot demethylate 3 and 4 to form 1 and 2. Increased estrogenicity of aboveground plant material collected on 10/18/01 is likely due to the increase in 2 concurrently observed, which may be due to bioconversion of 4 into 2 in the plant.
To follow up on the idea that different cell lines commonly used in estrogenic assays may have varying ability to metabolize isoflavones and effect estrogenic responses, we also tested the red clover extracts in an estrogen receptor-positive MCF-7 breast cell proliferation assay, at the same final concentration as that used in the Ishikawa cell assay. Although we measured different “estrogenic” responses, one can observe dramatic differences in the patterns of growth response of the MCF-7 cells compared with the AP induction response of the Ishikawa cells illustrated in Figures 3 and 4. It is likely that the extracts are acting as estrogens in the MCF-7 cells, rather than simply augmenting cell growth via other mechanisms, since co-incubation of 10 μg/mL of the red clover extracts with 10 μM tamoxifen citrate partially attenuates their ability to induce cell growth (data not shown). Although we could map the Ishikawa AP response directly onto the daidzein + genistein chemical content pattern of the extracts, this same pattern is not evident for the MCF-7 results. Additionally, the MCF-7 cell growth response was much lower on 9/04/01 (aboveground parts) and 9/18/01 (flower heads), compared with the rest of the collection dates. The extract of aboveground parts from 9/04/01 contained lower levels of daidzein + genistein relative to all the other aboveground part extracts. However, the extract of flower heads from 9/18/01 had similar or higher individual isoflavone content relative to the other flower head extracts, so we cannot explain this outlying response, unless perhaps an undetected antiestrogenic compound or plant contaminant was present in this sample of flower heads. None of the extracts were cytotoxic to MCF-7 cells. MCF-7 cells have been reported to convert biochanin A into genistein (23), but it stands unknown whether they also can produce daidzein from formononetin or equol from daidzein. The latter transformation would be especially important to know, considering that equol is known to effect many estrogenic responses in MCF-7 cells, including growth stimulation, estrogen receptor (ER) binding, and downregulation of ER mRNA (24–25).
Our data indicate that some caution should be exercised when evaluating botanical supplements or plants according to in vitro estrogenic assays, as cell-based experiments do not always account for the metabolic activation of isoflavones that has been demonstrated to occur in vivo. In vivo, 3 and 4 are metabolically converted into 1 and 2, respectively, and 1 can undergo further transformation in humans to form equol, which is also estrogenic (26). Equol is not normally found in red clover plants outright, as it is a human (gut flora) metabolite, and so it cannot be measured in a botanical supplement. Additionally, other compounds present in red clover extracts, such as prunetin, are metabolically converted into 2 (27). In the wider picture, using in vitro assays to screen agricultural plant samples for biological activity may be problematic due to this metabolic complexity. This idea should be considered by individuals who are formulating guidelines for the research and manufacture of botanical dietary supplements that are to be “biologically standardized.” It may be possible to overcome this metabolic bioassay “hurdle” by developing in vitro cell- or enzyme-based systems that are capable of metabolically activating extracts, while simultaneously measuring their biological activity (28).
Acknowledgments
NLB gratefully acknowledges Dr. Jimmy Orjala for his generous donation of HPLC instrument time for analysis of the red clover extracts.
Footnotes
FINANCIAL SUPPORT This work was supported, in part, by grant P50 AT00155 provided jointly by the National Center for Complementary and Alternative Medicine (NCCAM), the Office of Dietary Supplements (ODS), the Office for Research on Women’s Health (ORWH), and the National Institute of General Medicine (NIGMS) of the National Institutes of Health (NIH). NLB is grateful for a National Research Service Award (NRSA) from NCCAM, F31 AT00804. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, NCI, ODS, ORWH, NIGMS, or NIH.
Contributor Information
Nancy L. Booth, UIC/NIH Center for Botanical Dietary Supplements Research, Program for Collaborative Research in the Pharmaceutical Sciences (PCRPS) and Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, University of Illinois at Chicago, 833 S. Wood St., M/C 781, Chicago, IL 60612
Cassia R. Overk, UIC/NIH Center for Botanical Dietary Supplements Research, Program for Collaborative Research in the Pharmaceutical Sciences (PCRPS) and Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, University of Illinois at Chicago, 833 S. Wood St., M/C 781, Chicago, IL 60612
Ping Yao, UIC/NIH Center for Botanical Dietary Supplements Research, Program for Collaborative Research in the Pharmaceutical Sciences (PCRPS) and Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, University of Illinois at Chicago, 833 S. Wood St., M/C 781, Chicago, IL 60612.
Steve Totura, UIC/NIH Center for Botanical Dietary Supplements Research, Program for Collaborative Research in the Pharmaceutical Sciences (PCRPS) and Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, University of Illinois at Chicago, 833 S. Wood St., M/C 781, Chicago, IL 60612.
Yunfan Deng, Department of Mathematics, Statistics, and Computer Science, College of Arts and Sciences, University of Illinois at Chicago, 851 S. Morgan St., M/C 249, Chicago, IL 60607.
A. S. Hedayat, Department of Mathematics, Statistics, and Computer Science, College of Arts and Sciences, University of Illinois at Chicago, 851 S. Morgan St., M/C 249, Chicago, IL 60607
Judy L. Bolton, UIC/NIH Center for Botanical Dietary Supplements Research, Program for Collaborative Research in the Pharmaceutical Sciences (PCRPS) and Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, University of Illinois at Chicago, 833 S. Wood St., M/C 781, Chicago, IL 60612
Guido F. Pauli, UIC/NIH Center for Botanical Dietary Supplements Research, Program for Collaborative Research in the Pharmaceutical Sciences (PCRPS) and Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, University of Illinois at Chicago, 833 S. Wood St., M/C 781, Chicago, IL 60612
Norman R. Farnsworth, UIC/NIH Center for Botanical Dietary Supplements Research, Program for Collaborative Research in the Pharmaceutical Sciences (PCRPS) and Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, University of Illinois at Chicago, 833 S. Wood St., M/C 781, Chicago, IL 60612
LITERATURE CITED
- 1.United States Department of Agriculture. [Accessed December 07, 2004]; website://plants.usda.gov/index.html.
- 2.Wall OA. Handbook of Pharmacognosy. C. V. Mosby Company; St Louis, MO: 1917. pp. 384–385. [Google Scholar]
- 3.Hartwell JL. Plants Used Against Cancer, A Survey. Quarterman Publications, Inc.; Lawrence, MA: 1982. pp. 311–313. [Google Scholar]
- 4.Grieve MA. A Modern Herbal. Dover Publications, Inc.; New York, NY: 1978. pp. 207–208. [Google Scholar]
- 5.Hussey JS. Some useful plants of early New England. Econ Bot. 1974;28:311–337. [Google Scholar]
- 6.Newcomb EL. Kraemer’s Scientific and Applied Pharmacognosy. 3. John Wiley & Sons, Inc.; New York, NY: 1928. pp. 391–392. [Google Scholar]
- 7.Herrick JW. Dissertation. The State University of New York; Albany: 1977. Iroquois Medical Botany. [Google Scholar]
- 8.Bennetts HW, Underwood EJ, Shier FL. A specific breeding problem of sheep on subterranean clover pastures in western Australia. Aust Vet J. 1946;22:2–12. doi: 10.1111/j.1751-0813.1946.tb15473.x. [DOI] [PubMed] [Google Scholar]
- 9.Adams NR. Permanent infertility in ewes exposed to plant oestrogens. Aust Vet J. 1990;67:197–201. doi: 10.1111/j.1751-0813.1990.tb07758.x. [DOI] [PubMed] [Google Scholar]
- 10.Vetter J. Isoflavones in different parts of common Trifolium species. J Agric Food Chem. 1995;43:106–108. [Google Scholar]
- 11.Francis CM, Millington AJ, Bailey ET. The distribution of oestrogenic isoflavones in the genus Trifolium. Aust J Agric Res. 1967;18:47–54. [Google Scholar]
- 12.Pieterse PJS, Andrews FN. The estrogenic activity of alfalfa and other feedstuffs. J Anim Sci. 1956;15:25–36. [Google Scholar]
- 13.Cheng E, Burroughs W. In: American Association for the Advancement of Science. Sprague HB, editor. Vol. 53. Grasslands, New York: 1956. pp. 195–201. [Google Scholar]
- 14.Manda T, Matsumoto T, Sato K. Studies on estrogenic substances in herbage. III. Seasonal and yearly variations of estrogenic activity of legumes. J Japan Grassl Sci. 1971;17:205–211. [Google Scholar]
- 15.Flux DS, Munford RE, Wilson GF. Biological estimation of oestrogenic activity in red clover (Trifolium pratense): relative potencies of parts of plant and changes with storage. J Dairy Sci. 1963;30:243–249. [Google Scholar]
- 16.Vetter JCSA, Nagy G. Comparative study of estrogenic isoflavones in the genus Trifolium. Botanikai Kozlemenyek. 1991;78:137–149. [Google Scholar]
- 17.Dedio W, Clark KW. Biochanin A and formononetin content in red clover varieties at several maturity stages. Can J Plant Sci. 1968;48:175–181. [Google Scholar]
- 18.Kallela K, Saastamoinen I, Huokuna E. Variations in the content of plant oestrogens in red clover-timothy-grass during the growing season. Acta Vet Scand. 1987;28:255–262. doi: 10.1186/BF03548591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beck AB. The oestrogenic isoflavones of subterranean clover. Aust J Agric Res. 1964;15:223–230. [Google Scholar]
- 20.Pisha E, Pezzuto JM. Cell-based assay for the determination of estrogenic and anti-estrogenic activities. Methods Cell Sci. 1997;19:37–43. [Google Scholar]
- 21.Liu J, Burdette JE, Xu H, Gu C, van Breemen RB, Bhat KP, Booth N, Constantinou AI, Pezzuto JM, Fong HH, Farnsworth NR, Bolton JL. Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J Agric Food Chem. 2001;49:2472–2479. doi: 10.1021/jf0014157. [DOI] [PubMed] [Google Scholar]
- 22.Heinonen SM, Wahala K, Adlercreutz H. Identification of urinary metabolites of the red clover isoflavones formononetin and biochanin A in human subjects. J Agric Food Chem. 2004;52:6802–6809. doi: 10.1021/jf0492767. [DOI] [PubMed] [Google Scholar]
- 23.Peterson TG, Coward L, Kirk M, Falany CN, Barnes S. The role of metabolism in mammary epithelial cell growth inhibition by the isoflavones genistein and biochanin A. Carcinogenesis. 1996;17:1861–1869. doi: 10.1093/carcin/17.9.1861. [DOI] [PubMed] [Google Scholar]
- 24.Welshons WV, Murphy CS, Koch R, Calaf G, Jordan VC. Stimulation of breast cancer cells in vitro by the environmental estrogen enterolactone and the phytoestrogen equol. Breast Cancer Res Treat. 1987;10:169–175. doi: 10.1007/BF01810580. [DOI] [PubMed] [Google Scholar]
- 25.Sathyamoorthy N, Wang TTY. Differential effects of dietary phyto-oestrogens daidzein and equol on human breast cancer MCF-7 cells. Europ J Cancer. 1997;33:2384–2389. doi: 10.1016/s0959-8049(97)00303-1. [DOI] [PubMed] [Google Scholar]
- 26.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 27.Hu M, Krausz K, Chen J, Ge X, Li J, Gelboin HL, Gonzalez FJ. Identification of CYP1A2 as the main isoform for the Phase I hydroxylated metabolism of genistein and a prodrug converting enzyme of methylated isoflavones. Drug Metab Dispos. 2003;31:924–931. doi: 10.1124/dmd.31.7.924. [DOI] [PubMed] [Google Scholar]
- 28.Bennetau-Pelissero C, Latonnelle KG, Lamothe V, Shinkaruk-Poix S, Kaushik SJ. Screening for oestrogenic activity of plant and food extracts using in vitro trout hepatocyte cultures. Phytochem Anal. 2004;15:40–45. doi: 10.1002/pca.741. [DOI] [PubMed] [Google Scholar]


