Abstract
Objectives
To assess self-reported side effects in women after starting the OC, and to compare discontinuation rates according to presence or absence of side effects.
Study design
1716 women aged less than 25 years initiated the OC at 3 publicly funded family planning clinics and completed structured interviews after 3 and 6 months.
Results
Nearly 60% of subjects discontinued the OC by 6 months. Most subjects reported no changes in headaches, weight, moodiness, and sexual satisfaction during the first 3 months of OC use. Subjects with any complaints, especially those with increased headaches or moodiness, were more likely to discontinue the OC prematurely. Nonetheless, most discontinuation occurred for reasons unrelated to side effects.
Conclusions
Side effects are absent or mild among most OC users, but women with complaints are more likely to discontinue. Side effects are a less important reason for discontinuation than widely believed.
Keywords: Oral contraceptives, side effects, continuation rates
Oral contraceptives (OC) are highly effective, safe and widely used; approximately 85% of U.S. women will use the OC for an average of five years.1 However, women's OC use, similar to other chronic medications, is often inconsistent and transient.2 Reported six-month OC discontinuation rates vary from 18% to 50%.3,4,5 Unintended pregnancy often follows OC discontinuation because the discontinuers remain at risk of pregnancy, but do not obtain another highly effective birth control method.
Side effects are the most commonly reported reason why women discontinue using the OC. In a cross-sectional survey of 6676 European women, participants identified side effects as the most common reason for discontinuing the OC.6 Women in that survey who reported past side effects were about twice as likely to have discontinued the OC as women who reported not experiencing side effects. The value of this comparison is limited, however, by the cross-sectional and retrospective nature of the data collection. In a prospective cohort study of 1657 U.S. women enrolled from family planning clinics and private practices, 37% of OC users who discontinued cited side effects as the reason for discontinuation; however, this study had no information regarding the prevalence of side effects among the women who continued OC use.7
In our study of OC initiation and continuation, we asked all participants at three months and again at six months about any changes in the occurrence of or intensity of specific symptoms. We chose to examine only symptoms that many clinicians and patients view as OC side effects: weight change, headaches, mood changes, and sexual satisfaction. We also asked the subjects whether they attributed these symptoms to the OC or to other causes. Finally, we asked all discontinuers to identify reasons for discontinuation. This analysis compares these four specific symptoms among women who discontinue the OC during the first six months of use to those among women who continue the OC.
METHODS
This multi-center randomized trial was conducted between March 2003 and February 2005 at three University centers: Emory University, Atlanta, Mt. Sinai Medical Center, New York, and the University of Texas Southwestern, Dallas. Recruitment took place in their Family Planning and Teen Clinics. These clinics serve predominantly Latina and African-American populations; they are funded in part by Title X, as well as by other federal and state grants.
Clinical Center Institutional Review Boards and the Columbia University (coordinating center) IRB reviewed and approved the study. The study intervention was random assignment to immediate initiation of the OC at the time of study enrollment (Quick Start [QS], intervention group) or conventional initiation of the OC with the next menstrual period (Conventional Start [CS], control group).8 The results of the intervention and further details regarding study methods are reported elsewhere.9
Young women requesting oral contraceptives were the target population for recruitment in this study. After completion of clinical care following routine local protocols, health care providers referred women requesting the OC to study staff for screening according to the following eligibility criteria (1) aged less than 25 years, (2) requesting the OC as their primary method of contraception, (3) a negative pregnancy test on the day of enrollment, and (4) sexually active. Exclusion criteria included (1) medical contraindications to oral contraceptives, (2) current, continuing OC use, (3) use of Depo-Provera within the last 6 months, and (4) desiring pregnancy within the next 6 months.
After consent, eligible subjects participated in a baseline interview to collect demographic characteristics, sexual, contraceptive and reproductive history, and detailed contact information for subsequent interviews. After completion of the baseline interview, subjects underwent randomization. Treatment assignments were either Quick Start (QS) or Conventional Start (CS) of the OC in a 1:1 allocation ratio. All subjects received standardized pill-taking instructions along with a take-home instruction sheet. All study materials were available in English and Spanish and study staff were bilingual.
Subjects participated in three-month and six-month telephone interviews to assess OC continuation, pregnancy since date of last interview, sexual activity (i.e., risk of pregnancy), and adverse events as well as the full range of attitudes and behaviors addressed at baseline. Women who were already pregnant at the time of the three month interview (n=84) were not eligible for the six month interview. Follow-up interviews included specific questions regarding symptoms considered to be OC side effects, and questions regarding reasons for OC discontinuation. We developed the interview questions using focus groups at all of the clinics that participated in the study. We then carried out 300 pilot tests of the questionnaires prior to initiation of the trial.
All subjects who underwent follow-up interviews received the same sequence of questions about possible side effects regardless of whether they continued or discontinued the OC. We prefaced these questions with the statement "The following are some experiences that many women have, whether or not they take the pill. These can be either good or bad experiences." The first question asked, "Since the previous interview, have you gained weight, lost weight, or had no change at all in your weight?" If the subjects indicated a change, we then asked "did you experience a little change, some change, or a lot of change?" We then asked "Was it a good change, a bad change, or neither?" Finally, we asked "Do you think the change was mostly due to the pill or mostly due to something else?" After the sequence of questions regarding weight change, we asked about headaches, vision changes (as a control variable, unrelated to OC use), sexual satisfaction, and moodiness. For each symptom we used the same sequence of questions. For each symptom we combined the responses into a scale from −3 to +3, where −3 indicates a lot of change in a negative direction, 0 indicates no change, and +3 indicates a lot of change in a positive direction. We also dichotomized the responses regarding each symptom into no change or improved symptoms versus worsened symptoms. Finally, among women with worsened symptoms, we subdivided these according to whether the subjects believed her symptoms changes were due to the OC or due to some other cause.
At the end of follow-up, we classified participants as OC continuers or discontinuers. We defined OC continuation as being a continuous and current (that is, having taken a pill within the past seven days) OC user at the time of the follow-up interviews. Women who had not taken any OC for greater than 7 days prior to the interview (not counting the placebo pills) were classified as discontinuers. We chose the 7-day threshold based on sensitivity analyses in our pilot studies. We asked all discontinuers open ended questions about their reasons for stopping; for the few subjects who offered more than one reason, we elicited a main reason. We then individually evaluated and coded all reasons offered by the subjects; finally, we combined the many individual reasons for stopping into three categories: logistic reasons, side effects, and other. The category of logistic reasons included problems such as running out of pills, being unable to get back to the clinic, and being unable to afford more pills.
This analysis assesses whether OC discontinuers report more negative side effects (at 3 months) than women who continue the OC for at least six months. We compared the mean side effect intensities using the Mann-Whitney U test. We also compared whether OC initiators who report specific side effects have greater six-month OC discontinuation rates than women who report fewer or less intense side effects, calculating odds ratios with 95% confidence intervals. We used logistic regression to evaluate the impact of side effects and other covariates on OC continuation.
RESULTS
We enrolled 1720 women in the study. Four women enrolled twice and we excluded their second enrollment, leaving 1716 women. Subjects were mainly Hispanic, young and poor; about 40% had previous episodes of oral contraceptive use (Table 1). Overall, 89% of eligible subjects completed the 3-month interview (n=1498). Subsequently, 84% percent of eligible subjects completed the 6-month interview (n=1369). Ten percent of the subjects (n=171) were pregnant during follow-up. There were 97 subjects (5.6%) who missed both follow-up interviews and contributed only baseline information; these subjects were less likely to enroll at the Texas clinic, but otherwise resembled the subjects who had follow-up data. There were 1281 subjects (75% of all those who enrolled) who underwent both 3-month and 6-month interviews; their 3-month symptoms and 6-month OC continuation status inform the main analyses presented here. Subjects who changed to another contraceptive method during follow-up are included in this analysis as OC discontinuers. The OC continuation rate was 61% at the three month interview and 43% at the six month interview.
Table 1.
Baseline characteristics
| Baseline characteristics | Total enrolled n (%) |
|---|---|
| Total | 1716 (100%) |
| Site | |
| Emory | 325 (18.9%) |
| Mt. Sinai | 634 (36.9%) |
| Texas | 757 (44.2%) |
| Race | |
| Black | 612 (35.7%) |
| Hispanic | 1007 (58.7%) |
| Other | 97 (5.6%) |
| Age | |
| Under 18 | 566 (33%) |
| 18 or older | 1150 (67%) |
| Education | |
| High school grad/GED | 617 (36%) |
| No High school grad/GED | 1099 (64%) |
| Financial insecurity in past 6 months (n=1713) | |
| % with 1 or more needy experiences | 445 (25.9%) |
| no past experiences of financial need | 1268 (74.1) |
| Relationship status (n=1596) | |
| Neither married nor living together | 959 (60.1%) |
| Living together | 380 (23.8%) |
| Married | 257 (16.1%) |
| Previous OC use (%) | 695 (40.5%) |
| Previous Satisfaction with the Pill (n=688) | |
| Very unsatisfied | 39 (5.7%) |
| Somewhat unsatisfied | 35 (5.1%) |
| Somewhat satisfied | 150 (21.8%) |
| Very satisfied | 464 (67.4%) |
| Previous Pregnancies (%) | 991 (58.4%) |
| Number of past pregnancies (n=1698) | |
| None | 707 (41.6%) |
| One | 589 (34.7%) |
| Two or more | 402 (23.7%) |
| Pregnancies that were unplanned (%) | 68.7 ± 43.6% |
| Feeling about pregnancy in the next 6 months (n=1708) | |
| Very unhappy | 738 (43.2%) |
| Somewhat unhappy | 443 (25.9%) |
| Somewhat happy | 428 (25.1%) |
| Very happy | 99 (5.8%) |
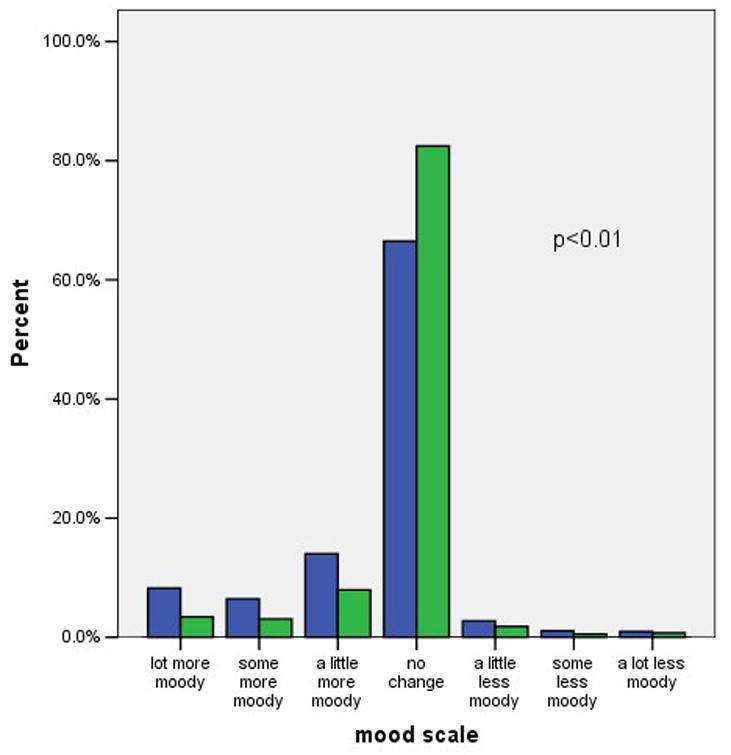
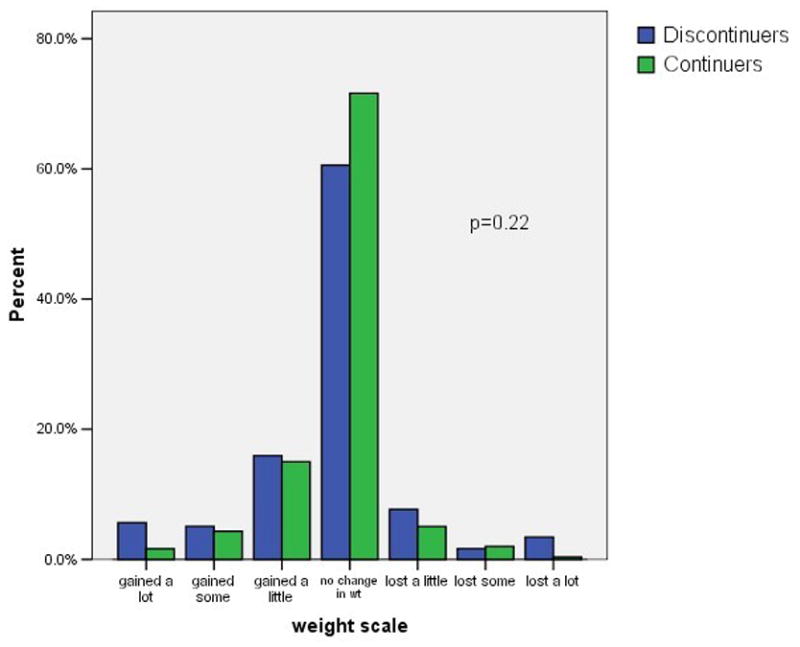
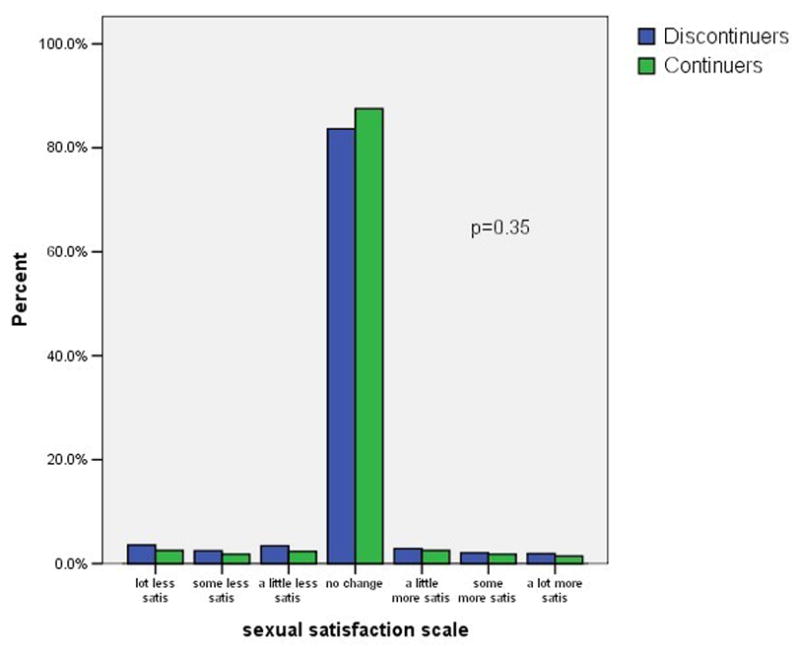
Figure 1 shows the distribution of self-reported weight changes, headaches, moodiness, sexual satisfaction, and visual changes at three months after initiating the pill. We included visual changes as a control variable, unrelated to OC use. Negative scores indicate negative changes such as weight gain or increased headaches. Positive scores indicate positive changes such as weight loss or decreased moodiness. Possible scores for individual items range from −3 to +3. A maximum score of −3 indicates a large negative change, and a score of +3 indicate a large positive change. A score of zero indicates no change. As indicated in the figure most subjects reported little or no change in these five factors. The difference in symptom scores between continuers and discontinuers was clinically modest, but statistically significant, for headache and moodiness. In contrast, there was not a statistically significant difference in the scores regarding weight gain or decreased sexual satisfaction.
Figure 1. Symptom scores among OC continuers and discontinuers.
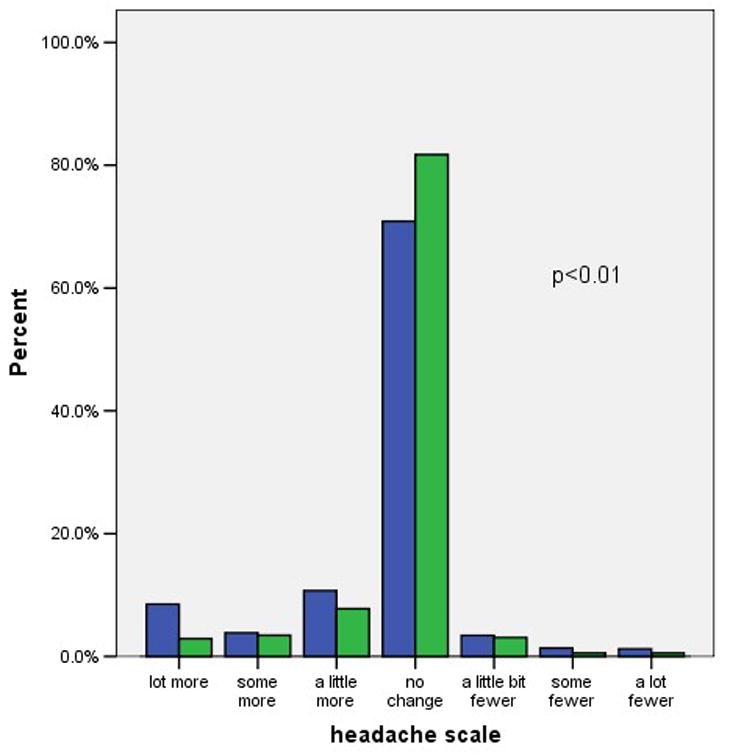
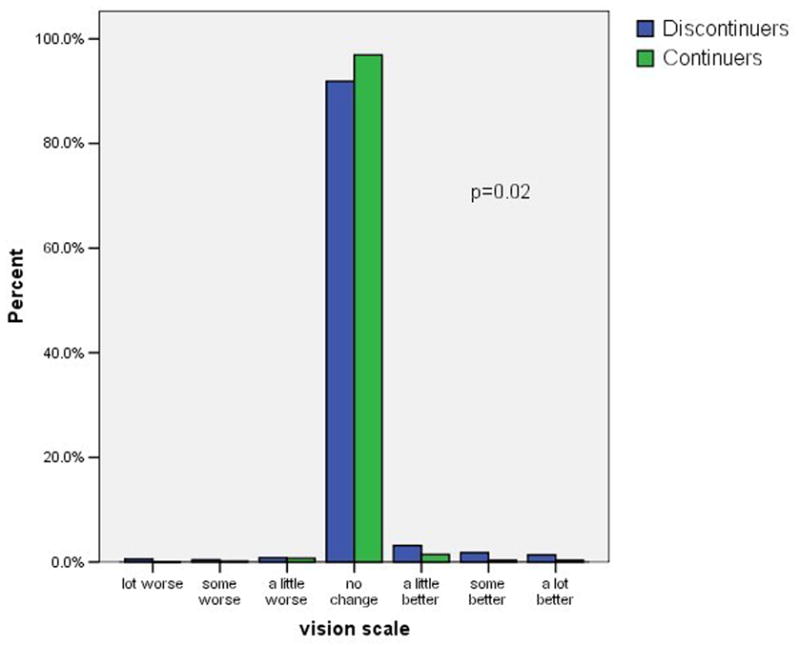
1a. Headache
1b. Moodiness
1c. Weight
1d. Sexual satisfaction
1e. Vision
footnote: p-values from Mann-Whitney U test comparing symptoms scores in continuers versus discontinuers.
Table 2 shows the impact of negative symptoms at 3 months on OC continuation rates at 6 months. We subdivided the negative symptoms according to whether the subject attributed these changes to the OC or to other factors. Weight change was the only symptom for which a "bad" change occurred in both directions, that is, some categorized gaining weight as a "bad" change and some characterized losing weight as a "bad" change; therefore, we present the continuation outcomes for weight gain and for "bad" weight change separately. The "symptom summary" variable combines all 654 subjects who reported no negative symptoms and compares them to those who reported at least one negative symptom. Results for OC discontinuation at 3 months were similar (data not shown).
Table 2.
3 month symptom changes and continuing the OC at 6 months
| Continuing OC at 6 months | |||
|---|---|---|---|
| N | % | OR (95% CI) | |
| Total | 1281* | 43.2% | |
| Self-reported symptom change at 3-month interview | |||
| Headache | |||
| -unchanged/less frequent | 1081 | 45.4% | 1.0 |
| -increased (attributed to pill) | 134 | 30.6% | 0.5 (0.4; 0.8) |
| -increased (attributed to other) | 42 | 26.2% | 0.4 (0.2; 0.8) |
| Moodiness | |||
| -unchanged/less moody | 1111 | 45.5% | 1.0 |
| -more moody (attributed to pill) | 89 | 27.0% | 0.4 (0.3; 0.7) |
| -more moody (attributed to other) | 55 | 32.7% | 0.6 (0.3; 1.0) |
| Weight | |||
| -unchanged/lost weight | 971 | 45.0% | 1.0 |
| -gained (attributed to pill) | 190 | 33.2% | 0.6 (0.4; 0.8) |
| -gained (attributed to other) | 92 | 40.2% | 0.8 (0.5; 1.2) |
| Weight | |||
| -unchanged | 957 | 46.1% | 1.0 |
| -"good" change | 152 | 38.2% | 0.6 (0.4, 0.8) |
| -"bad" change | 172 | 31.4% | 0.8 (0.5, 1.2) |
| Sexual Satisfaction | |||
| -unchanged/increased | 1232 | 43.3% | 1.0 |
| -decreased (attributed to pill) | 28 | 35.7% | 0.7 (0.3; 1.5) |
| -decreased (attributed to other) | 9 | 33.3% | 0.6 (0.2; 2.5) |
| Vision | |||
| -unchanged/improved | 1229 | 44.1% | 1.0 |
| -worse (attributed to pill) | 22 | 27.3% | 0.5 (0.2; 1.2) |
| -worse (attributed to other) | 24 | 12.5% | 0.2(0.05, 0.6) |
| Symptom Summary | |||
| -no negative changes | 654 | 51.1% | 1.0 |
| -any negative (attributed to pill) | 288 | 33.7% | 0.5 (0.4, 0.6) |
| -any negative (attributed to other) | 292 | 34.2% | 0.5 (0.4, 0.6) |
symptom change numbers do not all add to 1281 due to some missing responses
Overall, only 43% of these young subjects were current, continuous users of the OC at the 6-month interview. The women who reported no change or an improvement in the five symptoms we assessed were more likely to be continuing users than the women with at least one worsening symptom (51% versus 34%% respectively, p < 0.01). Decreased continuation rates occurred among women with each of the five symptoms that we considered. Remarkably, women who reported worsening vision, a symptom selected for inclusion here because it has no known relation to the OC, were even more likely to discontinue the OC than women reporting symptoms that are widely attributed to the OC. Half of the women reporting negative symptoms attributed these to the OC and half attributed the change to other factors. Attributing the change in symptoms to the OC rather than to other factors had no relation to the continuation rates. Adjusting these analyses for the main intervention (quick start versus conventional start), study site, age, previous OC use, and other factors related to OC continuation had no effect on the odds ratios shown in table 2.
If the 580 women with at least one of these particular symptoms had discontinued the OC at the same rate as the 654 women without these symptoms, there would have been 99 fewer OC discontinuations at 6 months; thus, these symptoms account for 14% of all discontinuations, and contribute about 6% to the overall 6-month discontinuation rate (the attributable risk fraction).
Figure 2 shows the reasons for OC discontinuation among all 1619 subjects who had at least one follow-up interview; thus, including some subjects who did not contribute to the previous analyses. Most discontinuers stopped the OC for logistic reasons. We categorized running out of pills, being unable to get back to the clinic, forgetting to take the pill, and similar problems related to either obtaining the pill or using it correctly as logistic reasons for stopping. Side effects were the next most common reason for stopping; side effects included the symptoms we had asked about specifically and menstrual changes, acne, and hair changes, as well as stating "I didn't like it". We also included fear of side effects in this category, but very few women in this study stated that fear of side effects was their reason for stopping. Finally, the remaining women offered other reasons for stopping – the "other" reasons were appropriate, and included (in order of frequency) no sexual activity, pregnancy, medical contraindication to the OC, and change to a different contraceptive method. We could not distinguish pregnancies associated with correct versus incorrect pill use, but the expected number of perfect-use pill failures would be very low. The women who reported stopping the OC due to side effects had more negative scores for the weight, headache, moodiness, and visual changes variables than the women who reported quitting for other reasons (p < 0.01 for all comparisons). In contrast, decreased sexual satisfaction was not associated with stopping due to side effects; more specifically, individual subjects did not report decreased sexual satisfaction as a reason for stopping the OC.
Figure 2.
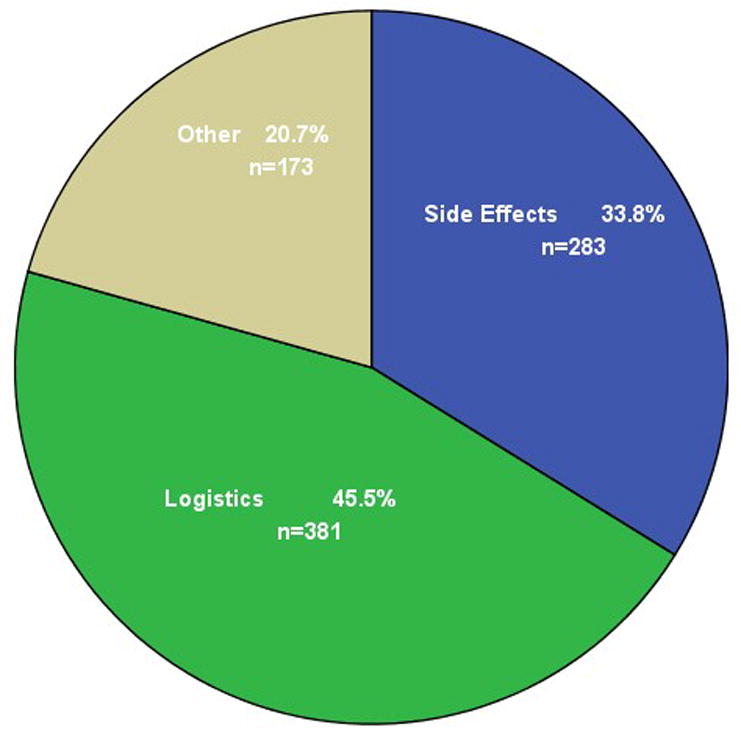
Main reason for stopping the OC
DISCUSSION
This is a large community-based study prospectively assessing side effects in women starting the OC. The 6-month discontinuation rate of 57% is comparable to smaller studies in similar patient populations.3,4,5 Our study is unusual in assessing symptoms in all OC users with a structured questionnaire. We chose four possible side effects to assess in detail – weight change, headaches, moodiness, and sexual satisfaction. These symptoms emerged as those most important to our patients during the preparatory focus groups that we carried out in the clinical centers that enrolled subjects in this study.10 We did not collect detailed information on other possible side effects that may be of interest to clinicians, such as breakthrough bleeding. We excluded evaluation of bleeding for two reasons: first, it was not of great importance to our patients in the focus groups; second, we were not confident that we could obtain high quality data regarding bleeding in our telephone interviews. We believe bleeding patterns are better assessed using more intensive follow-up than was possible in this study.11
We chose to present symptoms reported during the 3-month interview and their association with OC discontinuation at any time during the 6 month study. This allowed us to consider the maximum number of OC discontinuations that might have been related to symptoms present at 3 months. When we limited our analyses to early discontinuations only, the patterns that emerged were similar, but were based on fewer observations. We also collected 6-month symptom information; however, in many cases, those subjects were reporting on symptoms present after they already discontinued the OC. The associations were similar to those shown, but the correct interpretation is unclear. This may suggest that complaining of any symptoms, at any time, is a marker for OC discontinuation.
This study clearly shows that women who report increased headache and moodiness during early months of OC use are more likely to discontinue. Subjects who gained weight or reported a "bad" weight change were also more likely to discontinue the OC by six months. These data, however, do not show that the OC caused the reported symptom – in fact, about half of the subjects believed their symptoms occurred for a reason other than the OC. The subjects who attributed the negative symptom to other causes were just as likely to discontinue the pill as the subjects who attributed the negative symptom to the pill. The same associations are present, but weaker, for sexual satisfaction and changes in vision (our control variable). A possible interpretation of these associations is that women who have negative feelings of any kind, regardless of perceived cause, are more likely to discontinue their oral contraceptive. This interpretation is supported by our previous findings that women with higher depression scores at enrollment were significantly more likely to discontinue DMPA within six months.12 We have reported elsewhere that the use of DMPA or the OC does not worsen depression scores.13 We did not obtain a baseline depression score in this study, and therefore, cannot assess the contribution of depression to discontinuation in this study population.
Very little OC discontinuation can be attributed to OC side effects as defined in this study.14 The association between OC use and libido has received attention in the popular press; our results showing essentially no change in sexual satisfaction among our OC users may help to deflate this emerging myth. The most important factor leading to OC discontinuation in our subjects was not side effects, but difficulty in obtaining more pills and in using the pills correctly. These results suggest that improving ready access to the OC may have the biggest impact on method continuation in the poor, young, inner-city women who participated in this study. Reducing possible side effects is always desirable, but working on improved accessibility is likely to have more impact on OC continuation rates for poor women. The widespread focus on side effects may be a distraction from more important health services issues.
Footnotes
Condensation
Perceived side effects among OC users tend to be mild, are often similar in continuers and discontinuers, and explain only a minority of OC discontinuation.
This work was supported by NIH grant 1RO1-HD42413
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abma J, Chandra A, Mosher W, Peterson L, Piccinino L. Fertility, family planning, and women's health: New data from the 1995 National Survey of Family Growth. National Center for Health Statistics. Vital Health Stat. 1997:23. [PubMed] [Google Scholar]
- 2.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 3.Berenson AB, Wiemann CM. Contraceptive use among adolescent mothers at six months postpartum. Obstet Gynecol. 1997;89:999–100. doi: 10.1016/s0029-7844(97)00123-3. [DOI] [PubMed] [Google Scholar]
- 4.Emans SJ, Grace E, Woods ER, Smith DE, Klein K, Merola J. Adolescents’ compliance with the use of oral contraceptives. JAMA. 1987;257:3377–81. [PubMed] [Google Scholar]
- 5.Oakley D, Sereika S, Bogue E. Oral contraceptive pill use after an initial visit to a family planning clinic. Family Planning Perspectives. 1991;23:150–54. [PubMed] [Google Scholar]
- 6.Rosenberg MJ, Meehan TE, Waugh MS. Use and misuse of oral contraceptives: risk indicators for poor pill taking and discontinuation. Contraception. 1995;51:286–88. doi: 10.1016/0010-7824(95)00074-k. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg MJ, Waugh MS. Oral contraceptive discontinuation: a prospective evaluation of frequency and reasons. Am J Obstet Gynecol. 1998;179:577–82. doi: 10.1016/s0002-9378(98)70047-x. [DOI] [PubMed] [Google Scholar]
- 8.Westhoff C, Kerns J, Morroni C, Cushman L, Tiezzi L, Murphy PA. Quick start: a novel oral contraceptive initiation method. Contraception. 2002;66:141–5. doi: 10.1016/s0010-7824(02)00351-7. [DOI] [PubMed] [Google Scholar]
- 9.Westhoff C, Heartwell S, Edwards S, Zieman M, Cushman L, Kalmuss D. Oral Contraceptives: Quick Start Versus Conventional Start. Reproductive Health 2006; Association of Reproductive Health Professionals' (ARHP) 43rd Annual Conference; accepted. [Google Scholar]
- 10.Sneed R, Hutchko M, Robilotto T, Johnson S, Westhoff C. Using a health behavior model to predict oral contraceptive continuation. [Abstract] Contraception. 2003;68:139–55. [Google Scholar]
- 11.Westhoff C, Morroni C, Kerns J, Murphy PA. Bleeding patterns after immediate versus conventional oral contraceptive initiation: a randomized controlled trial. Fertility and Sterility. 2003;79:322–9. doi: 10.1016/s0015-0282(02)04680-0. [DOI] [PubMed] [Google Scholar]
- 12.Westhoff C, Truman C, Kalmuss D, Cushman L, Davidson A, Rulin M, Heartwell S. Depressive symptoms and Depo-Provera. Obstetrical & Gynecological Survey. 1998;53:695–696. doi: 10.1016/s0010-7824(98)00024-9. [DOI] [PubMed] [Google Scholar]
- 13.Davis A, O'Connell K. Oral contraceptives, side effects, and depression in adolescent girls. [submitted] [DOI] [PubMed] [Google Scholar]
- 14.Davis A, Schnare S. Hormonal contraceptives and libido. Dialogues in Contraception. 2005;9:5–8. [Google Scholar]


