Abstract
Previous studies have noted particular difficulty in achieving abstinence among those who are marijuana dependent. The present study employed a dismantling design to determine whether adding contingency management (ContM) to motivational enhancement therapy plus cognitive behavioral therapy (MET+CBT), an intervention used in prior studies of treatment for marijuana dependence, would enhance abstinence outcomes. 240 marijuana dependent participants were recruited via advertisements and assigned to either MET+CBT, ContM only, MET+CBT+ContM, or to a case-management control condition. All interventions involved 9 weekly 1-hour sessions, except for the ContM-only condition whose sessions lasted about 15 minutes. ContM provided reinforcement for marijuana-free urine specimens, in the form of vouchers redeemable for goods or services. Follow-up data were collected at posttreatment and at 3-month intervals for 1 year. The two ContM conditions had superior abstinence outcomes: ContM-only had the highest abstinence rates at posttreatment, and the MET+CBT+ContM combination had the highest rates at later follow-ups. The roles of contingency management and coping skills training in the treatment of marijuana dependence are discussed.
Keywords: Marijuana dependence, contingency management, cognitive-behavioral therapy, motivational enhancement therapy
1. Introduction
Marijuana is the most widely used illicit drug. Lifetime prevalence rates of cannabis dependence are estimated at 4% of the population (McRae, Budney & Brady, 2003). Long-term heavy use of marijuana increases the likelihood of depression and anxiety (Troisi, Pasini, Saracco & Spalleta, 1998), high risk sexual behavior (Bell, Wechsler & Johnston, 1997), and cognitive impairment (Solowij et al. 2002).
Despite the need for effective interventions, there has been relatively little controlled research evaluating treatment for marijuana use disorders (Moore & Budney, 2003). In the earliest controlled study, Stephens, Roffman, and Simpson (1994)compared relapse prevention to a support group, both of which reduced marijuana use and related problems, but only 14% maintained continuous abstinence throughout the follow-up year. In a subsequent study, Stephens, Roffman, and Curtin (2000)compared 14 sessions of relapse prevention, 2 sessions of motivational enhancement, and a delayed-treatment control. Both active treatments reduced marijuana use substantially, compared to the control group. At 4 months, 37% of those in the active interventions had 90 days of abstinence and at 7 months the 90-day abstinence rate was 34%, but the findings must be interpreted with caution because there was no verification of self-reported abstinence, and the clients were highly motivated for treatment, well educated, and socioeconomically advantaged.
The multi-site Marijuana Treatment Project (MTP) compared two interventions (2-session and 9-session) to a delayed treatment control, in a more heterogeneous sample (N=450). Nine sessions of combined Motivational Enhancement Therapy (MET) and Cognitive Behavioral Therapy (CBT) were superior to two sessions of MET, and both were superior to the control group (Marijuana Treatment Project Research Group, 2004). The highest abstinence rate was 23% at 4-month follow-up (9-session group), declining to 15% at 9 months. In another study, Moore and Budney (2003)concluded that marijuana-dependent clients have difficulty initiating and maintaining abstinence (see alsoMcRae et al., 2003).
In view of the difficulty achieving abstinence in those who are marijuana dependent, the present research sought to determine whether contingent reinforcement for marijuana-free urine specimens, when added to treatments used in some of the prior studies, would enhance abstinence outcomes. The underlying treatments were MET and CBT, as used in MTP (MTP Research Group, 2004). MET focuses on building motivation to change and resolving the ambivalence that often leads to attrition from marijuana treatment (Roffman, Klepsch, Wertz, Simpson & Stephens, 1993). CBT views substance dependence as learned behavior that is used to cope with problems or to meet needs, and therefore considers the acquisition of coping skills as essential (Monti, Kadden, Rohsenow, Cooney & Abrams, 2002). Reviews of alcohol and drug treatments concur that motivational enhancement and CBT skills training are effective interventions (DeRubeis & Crits-Christoph, 1998;Miller et al. 1995). The efficacy of combining motivational and skills treatment elements has been demonstrated for alcoholism (Allsop, Saunders, Phillips & Carr, 1997) and for marijuana dependence (Stephens & Roffman, 1996).
Contingency management (ContM) provides tangible reinforcement for abstinence and/or activities incompatible with drug use (Budney & Higgins, 1998;Higgins et al. 1994). It has been shown effective in promoting retention of clients in treatment (Higgins et al. 1994; Petry, Martin, Cooney & Kranzler, 2000), and promoting abstinence from cocaine (Higgins et al., 1994;Petry et al. 2004; Petry & Martin, 2005;Petry, Peirce et al. 2005), alcohol (Griffiths, Bigelow & Liebson, 1978;Petry et al. 2000), nicotine (Shoptaw, Jarvik, Ling & Rawson, 1996) and benzodiazepines (Stitzer, Bigelow, Liebson & Hawthorne, 1982). A recent meta-analysis showed that voucher-based reinforcement therapy generated better outcomes in 30 studies, involving a variety of abused substances, than control treatments (Lussier, Heil, Mongeon, Badger & Higgins, 2006).
However, there are few reports of ContM for marijuana dependence. A case report (Budney, Higgins, Delaney, Kent & Bickel, 1991) was the first to demonstrate that ContM could reduce marijuana use. A subsequent controlled study (Budney, Higgins, Radonovich & Novy, 2000) found that adding voucher-based incentives for marijuana abstinence resulted in longer periods of abstinence during treatment than did motivational and CBT interventions without ContM.
In an attempt to improve on the generally poor marijuana abstinence rates in prior studies, the present study added ContM to the combination of MET and CBT that had been utilized in prior studies. It was anticipated that the ContM component of the combined treatment would enhance both attendance and abstinence during treatment, resulting in better outcomes, as compared with ContM alone or MET+CBT without ContM.
A third comparison group received supportive case management (CaseM) to control for non-specific factors related to time spent in treatment. CaseM was selected on the basis of research suggesting the importance of identifying and reducing nonsubstance problems in the lives of drug users in order to achieve successful substance use outcomes (McLellan et al., 1997). In this approach, problems were identified, referrals made for needed services, and support provided for clients’ efforts to obtain those services.
Participants (N=240) were randomly assigned to 1 of 4 treatments, all of which lasted 9 sessions, in a dismantling design (seeTable 1):
Table 1. Design of the Study: Elements Tested in Each Treatment Condition.
| Treatment Condition | Treatment Elements Tested | |
|---|---|---|
| Coping skills enhancement | Reinforcement for abstinence | |
| 1. Case Management (CaseM: Active Control condition) | ||
| 2. MET+CBT | X | |
| 3. Contingency Management (ContM) | X | |
| 4. MET+CBT+ContM | X | X |
CaseM: an active control condition that focused on life issues such as occupational, social, psychiatric, or educational goals. It served as a control for time and attention, without teaching skills or tangibly reinforcing abstinence with anything other than verbal praise.
MET+CBT: this treatment, modeled after the 9-session treatment in MTP, taught skills for coping with high risk situations, to increase clients’ ability to remain abstinent.
ContM: This intervention provided reinforcement (vouchers redeemable for goods and services) for drug-free urine samples. Attendance was also reinforced, since vouchers could only be earned if participants came in to provide a urine specimen.
MET+CBT+ContM: This treatment combined MET+CBT with ContM reinforcement for drug-free urine samples. It was predicted that ContM would enhance the likelihood of achieving abstinence and that CBT would enhance the likelihood of maintaining abstinence.
It was predicted that the CaseM group would have the least abstinence, that the ContM-only and MET+CBT groups would have greater abstinence than CaseM, and that the MET+CBT+ContM group would have the best abstinence outcomes.
2. Methods
2.1 Subjects
Subjects were recruited through newspaper and radio advertisements seeking heavy marijuana users who had been unable to stop using on their own. Participants had to be at least 18 years old, meet DSM-IV criteria for Cannabis Dependence, and willing to accept random assignment to any of the four conditions. Exclusion criteria were acute medical or psychiatric problems that required inpatient treatment (e.g., acute psychosis, or serious suicide/homicide risk), current dependence on alcohol or other drugs, reading ability below the fifth grade level, lack of reliable transportation to the treatment site, or excessive commuting distance.
Sample size was determined by the power available to detect between-group differences on two outcome variables: proportion of days of marijuana use and proportion of participants reporting continuous abstinence, using procedures described byCohen (1988). Based on estimates of effect sizes and possible loss of subjects over the course of the study, it was estimated that 62 persons per treatment group would be required, or a total N of 248. We enrolled 240 subjects out of 606 who were screened. This left us with a sample sufficient to detect between-group differences in continuous abstinence at the last follow-up with a power just under .80, and alpha (1-tailed) set at .05. The primary reasons for loss of recruits were their lack of interest (failure to keep appointments or return calls), or unwillingness to be randomized. The main reasons for excluding people were concurrent alcohol or drug dependence, need for more intensive treatment, residential instability or living too far from the treatment center, frequent urine assays in another setting, not being diagnosed as marijuana dependent, or heavy involvement in self-help meetings.Figure 1 shows participant flow through the study from the initial telephone screening to the final assessment one year post-treatment, including numbers excluded for various reasons, reasons for dropout prior to randomization, and numbers of dropouts during treatment and the follow-up year.
Figure 1.
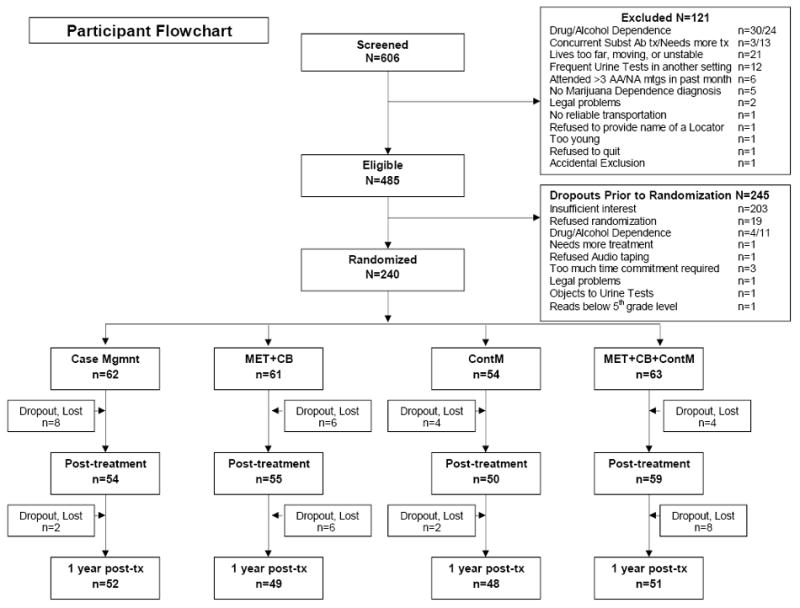
Participant flowchart showing numbers and reasons for exclusions and pre-randomization dropouts, numbers of participants assigned to each intervention, numbers of dropouts in each group, and numbers interviewed at the post-treatment and one-year follow-up assessment points.
2.2 Assessments
Individuals who responded to the ads were given a 20-minute Quick Screen interview by phone to identify those likely to meet the inclusion criteria, and to collect basic demographic and clinical information about the population from which we were sampling.
At the subsequent in-person interview, the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient edition, version 2.0 (SCID-I/P; First, Spitzer, Gibbon & Williams, 1996) was used to determine whether subjects met inclusion/exclusion criteria for cannabis abuse or dependence, alcohol or other drug dependence, and psychotic symptoms, during the past 90 days.
The Time Line Follow-Back (TLFB) interview gathered marijuana, other drug, and alcohol frequency-of-use data for the 90 days prior to intake and each follow-up. A calendar was used to determine the number of days on which consumption occurred (Sobell et al., 1980). The TLFB has good test-retest reliability, and validity for verifiable events (Sobell & Sobell, 1992).
The Marijuana Problems Scale was used to assess marijuana-related problems. It is based on items from the Drug Abuse Screening Test plus marijuana-specific items (Stephens, Wertz & Roffman, 1993). It has been shown to be sensitive to the effects of treatment (Marijuana Treatment Project Research Group, 2004), and at our site in MTP it had an internal reliability of alpha = .81.
The Addiction Severity Index (ASI), a structured interview for research and clinical settings (McLellan, et al., 1992), has been shown to be reliable and valid (McLellan et al., 1985). An abbreviated version, produced by the instrument’s developers, was administered at intake and at each in-person follow-up assessment.
Urine tests were performed at intake, prior to each intervention session, and at the in-person follow-ups, using EZ-Screen (Editek Inc., Burlington, NC), an onsite testing system that provides immediate qualitative results. The intake specimen was assessed for marijuana, cocaine, amphetamines, and opiates, and subsequent specimens for marijuana only. As a further check on marijuana abstinence between weekly treatment sessions, after three consecutive negative urines, clients were called in for an extra urine check between sessions.
2.3 Therapists and Research Assistants
Three of the therapists were licensed clinical psychologists, and the fourth had an MS in Family Studies and was a Certified Alcohol and Drug Abuse Counselor. They were provided training in the MET+CBT, MET+CBT+ContM, and CaseM treatment manuals. They then received weekly supervision for three training cases, one for each of those interventions, before being assigned actual study participants. Thereafter, ongoing supervision to assure treatment fidelity was biweekly, based on the clinical supervisor’s review of randomly selected treatment session tapes (36% of sessions were reviewed). The BA-level Research Assistants (RAs) were trained in the intake and follow-up assessments and in the ContM-only intervention, which they administered. The research assistants practiced all assessment and contingency management procedures on training cases prior to the enrollment of main phase participants .
2.4 Procedures
Prospective participants were first evaluated by telephone, and either scheduled for an intake interview or referred elsewhere for treatment. The final decision about eligibility was made at an in-person interview, based on the SCID-I/P (to determine presence of a cannabis dependence diagnosis, and of any exclusionary diagnoses). Those who were eligible and agreed to random treatment assignment completed an IRB-approved Informed Consent process. Participants were asked to name a ”significant other” who could provide information regarding their whereabouts if they moved without notice. Finally, participants were assigned to treatment by a computerized urn randomization process (Stout, Wirtz, Carbonari & DelBoca, 1994) that balanced the four treatment groups on gender, age, education level, ethnicity, employment status, and number of marijuana problems.
2.4.1 Treatment Interventions
All the interventions, except ContM-only, were conducted in 60-minute individual outpatient sessions, employing manuals that provided specific guidelines to the therapists. All four interventions were provided free of charge, and participants received a $5 gift certificate for attending the first session.
The MET+CBT intervention consisted of two sessions of motivational enhancement therapy followed by seven sessions of coping skills training, based on the therapy manual developed for MTP. The motivational enhancement component was derived from the Project MATCH MET manual (Miller, Zweben, DiClemente & Rychtarik, 1992). The MET approach involves an empathic style designed to help participants resolve ambivalence and develop motivation to change. The first session focused on a Personalized Feedback Report derived from the intake assessment, emphasizing problem areas that were identified. The second session focused on events that occurred since the first session, strengthening commitment to change and setting goals for behavior change.
In the third session, explicit CBT skills training commenced, to provide participants with means for coping with antecedents to marijuana use, based on a manual developed for alcoholism treatment research (Monti, Abrams, Kadden & Cooney, 1989). There were five ‘core’ sessions: 1) functional analysis of problems, 2) coping with cravings, 3) managing thoughts about marijuana use, 4) problem-solving, and 5) marijuana refusal skills. For the remaining two sessions, there was a choice from among five ‘electives’: assertiveness training, coping with anger, managing negative moods and depression, coping with emergency situations and relapse, or decision making.
ContM was both a stand-alone intervention and, in another condition, was combined with MET+CBT. Because ContM required verification of abstinence, participants in all conditions provided a urine specimen to be tested for marijuana at each treatment session. (In the MET+CBT condition the therapist informed participants of the weekly urine test results and if the test was positive suggested changes the participant could make; in CaseM participants were also informed of the test results, but no suggestions were made.) Participants in either of the ContM conditions received a voucher if they submitted a negative urine specimen. The ContM-only group did not receive any other treatment. They met for 9 weeks, for about 15 minutes, with an RA who collected a supervised urine specimen and managed the voucher system.
Because of the long period (20 or more days) in which marijuana may be detected in the urine of chronic users, vouchers were not available in Weeks 1 or 2, to avoid discouraging those who stopped using but nevertheless had a positive urine. Vouchers became available for marijuana-free urines, in the two ContM conditions, starting in Week 3.
The initial voucher rate was $10 for a clean urine in Week 3, and escalated by $15 per week for each successive marijuana-free urine specimen. A client who had all clean urines during the 7 weeks in which vouchers were available would receive a $100 voucher in the final week, and total earnings of $385. If a participant was positive for marijuana, no reward was given and the voucher value was reset to $10 for the next marijuana-negative urine. Once the participant submitted two consecutive negative urines ($10 + $25), values were reinstated to the highest level previously achieved. Vouchers could be redeemed for an immediate reward commensurate with their value, or be saved in a ‘bank’ for a larger reward when a number of vouchers were accumulated. Vouchers could be redeemed for retail goods or services that were not drug-related (Higgins et al. 1994; Higgins, Alessi & Dantona, 2002; Petry, Alessi, Tedford, Austin, & Tardif, 2005).
The CaseM intervention was supportive in nature. Clients were not provided suggestions regarding how to change marijuana use; rather, CaseM was designed to help participants with problems of daily living that may be due to, or contribute to, their marijuana use. During the course of 9 weekly sessions, the therapist and participant identified problems in daily living, established relevant goals, and considered personal and community resources that might be helpful (e.g., discussing problems with family members, or contacting a psychiatrist for depression). The therapist’s role was to explore clients’ concerns, provide encouragement and verbal support, and troubleshoot difficulties in obtaining services, in a context of supportive therapy. Efforts were made to minimize overlap with the MET+CBT and ContM interventions by explicitly avoiding motivational interviewing techniques and by not providing skills training or contingent tangible reinforcement.
2.4.2 Data collection
RAs conducted the intake and follow-up assessments. To prevent protocol drift, assessment interviews were audiotaped and a sample of them reviewed for protocol adherence. Participants were paid $50 for each in-person follow-up, and $20 for telephone follow-ups. If participants repeatedly failed to come for an in-person follow-up, they were offered a telephone interview. Failing that, the questionnaires were sent by mail. Of the in-person follow-ups scheduled, 4.5% were conducted by phone, and 1% via mail. All data were double entered for verification.
3. Results
3.1 Homogeneity of Treatment Groups
Chi-square analyses and one-way analyses of variance (ANOVA) were conducted to determine if the treatment groups were homogeneous with respect to baseline variables. For each treatment type the means or percentages for age, sex, race, education, employment, marijuana problems scale score, marijuana joints smoked per day, proportion of days abstinent in the 90 days prior to baseline assessment, and ASI subscales are shown inTable 2. None of the analyses yielded significant effects for Treatment condition, indicating that the treatment groups did not differ from one another with respect to these baseline variables.
Table 2. Baseline Characteristics of Sample.
| Variable | Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CaseM (n=62) | MET+CBT (n=61) | ContM (n=54) | MET+CBT+ContM (n=63) | Total (n=240) | ||||||
| M or % | SD | M or % | SD | M or % | SD | M or % | SD | M or % | SD | |
|
|
||||||||||
| Age | 31.9 | 9.6 | 34.1 | 7.8 | 33.4 | 11.4 | 31.8 | 9.6 | 32.7 | 9.6 |
| Sex (% Male) | 69 | -- | 72 | -- | 80 | -- | 64 | -- | 71 | -- |
| Race (% White) | 57 | -- | 56 | -- | 72 | -- | 59 | -- | 60 | -- |
| Years Education | 12.9 | 1.7 | 12.9 | 1.9 | 13.1 | 1.8 | 12.9 | 1.8 | 13.0 | 1.8 |
| Married (%) | 38.7 | -- | 44.3 | -- | 42.6 | -- | 55.6 | -- | 45.4 | -- |
| Employed (%) | 68 | -- | 82 | -- | 70 | -- | 73 | -- | 73 | -- |
| Marijuana Problems | 15.19 | 6.74 | 13.97 | 7.52 | 12.62 | 6.09 | 13.42 | 6.84 | 13.88 | 6.75 |
| Joints per Day | 5.2 | 5.7 | 4.67 | 6.27 | 3.24 | 2.65 | 4.76 | 3.98 | 4.50 | 4.93 |
| Proportion Days Abstinent | .08 | .12 | .08 | .13 | .15 | .19 | .11 | .17 | .11 | .15 |
| ASI Alcohol Composite | .09 | .10 | .12 | .12 | .11 | .14 | .09 | .09 | .10 | .12 |
| ASI Drug Composite | .26 | .05 | .25 | .07 | .23 | .07 | .23 | .07 | .24 | .07 |
| ASI Psychiatric Severity | .22 | .23 | .24 | .20 | .25 | .21 | .25 | .19 | .24 | .21 |
3.2 Attendance
A one-way ANOVA was conducted to determine if any of the treatments yielded more attendance. The mean number of sessions attended overall was 5.2 (SD = 3.5), with no treatment having superior attendance [F (3, 236) = 1.06;p > .36].
3.3 Frequency Outcome: Proportion of Days Abstinent (PDA)
Figure 2 shows PDA by treatment condition over all follow-up points for the 218 participants who provided data for at least one follow-up point. PDA increased at posttreatment (2-month outcome) in all conditions, and stayed elevated with no more than a relatively modest decline over the next 12 months in all but the ContM-only condition. Analyses of PDA were conducted using hierarchical linear regression models (HLM; Bryk & Raudenbush, 1992). This approach takes advantage of all available data by using a maximum likelihood estimation procedure to estimate the parameters of the multivariate normal regression model (Little and Rubin, 1987). The resulting estimates are highly reliable even if data are missing, particularly for large sample sizes (Schafer & Graham, 2002). In these analyses, terms were included as they would be in a conventional repeated measures ANOVA; the effects examined included Treatment, Time, and Treatment X Time. Treatment was considered as a fixed effect, and the intercept as a random effect. Time was calculated as time in months since baseline. An unstructured covariance matrix offered the best solution for the models based on residual log likelihood (RLL) and Aikaike’s Information Criteria (AIC). Results of the mixed procedure showed no main effect for Treatment, a significant effect for Time [F (5, 214) = 69.14;p < .001], and no significant Treatment X Time interaction.
Figure 2.
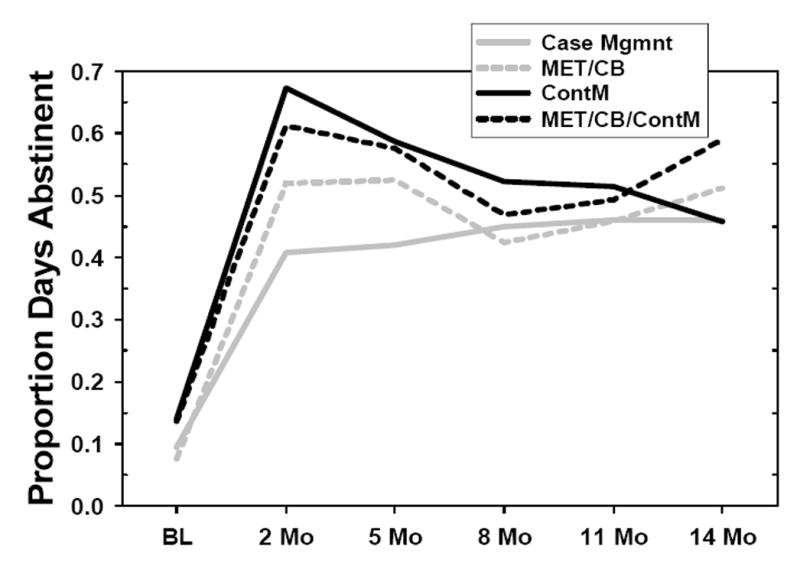
Proportion of Days Abstinent, by intervention, across all assessment points. BL=Baseline. All other assessments points are designated in months since the baseline assessment.
Planned contrasts were also conducted within the mixed model in order to examine the effects of MET+CBT and ContM separately, as well as the effect of the MET+CBT+ContM combination. The contrasts were specified as follows: (a) MET+CBT v. CaseM, (b) ContM v. CaseM, (c) MET+CBT+ContM v. CaseM, and (d) MET+CBT+ContM v. all other conditions. Of these contrasts, only the contrast of ContM with CaseM was significant [F (1, 214) = 4.13;p < .05]. Analyses of means indicated that subjects in ContM reported more days of abstinence than did those in CaseM. Analysis of simple effects indicated that this difference was significant only at the posttreatment time point, and not throughout the follow-up period.
3.4 Quantity Outcome: “Joints” Smoked Per Smoking Day
The number of marijuana units, or “joints”, smoked per smoking day was derived from the TLFB. Quantity of smoking declined for participants in all treatment groups. At baseline, clients were smoking an average of over 5 joints (approximately 1/8 ounce) per day, on days that they smoked. By posttreatment, they were smoking approximately 2.4 joints per day, and decreased to 1.7 per day at 5 months and to 0.5 per day at 14 months. As with the PDA outcome, the analysis of joints per day was conducted using mixed model regression, with the same constraints as described above. The data were log-transformed to correct for high positive skewness. Results of the mixed procedure showed no main effect for Treatment, a significant effect for Time [F (5, 214) = 152.66;p < .001], and no significant Treatment X Time interaction. Planned contrasts were conducted in the same manner as described for the PDA variable. None of the contrasts yielded significant results.
3.5 Continuous Abstinence Outcome
Although all treatments significantly increased the number of abstinent days, continuous abstinence is also considered an important outcome due to the risk of escalating use inherent in continued low-level marijuana use. Analysis of variance performed on the longest period of continuous abstinence (in days) by treatment condition revealed a significant linear contrast in which the MET+CBT, ContM, and MET+CBT+ContM conditions yielded progressively longer periods of continuous abstinence (tcontrast=2.12,df=215,p<.05) during follow-up, as shown inFigure 3. An additional significant contrast indicated that both ContM conditions resulted in significantly longer periods of continuous abstinence than did the two non-ContM conditions (tcontrast=2.00,df=215,p<.05). Finally, the ContM conditions resulted in continuous abstinence sooner than the non-ContM conditions (100 days after treatment entry vs. 160 days;p<.05). Reports of abstinence were validated by comparison with urinalysis results; measures of agreement ranged from kappa=.26 (posttreatment) to kappa=.8 (14-month follow-up).
Figure 3.
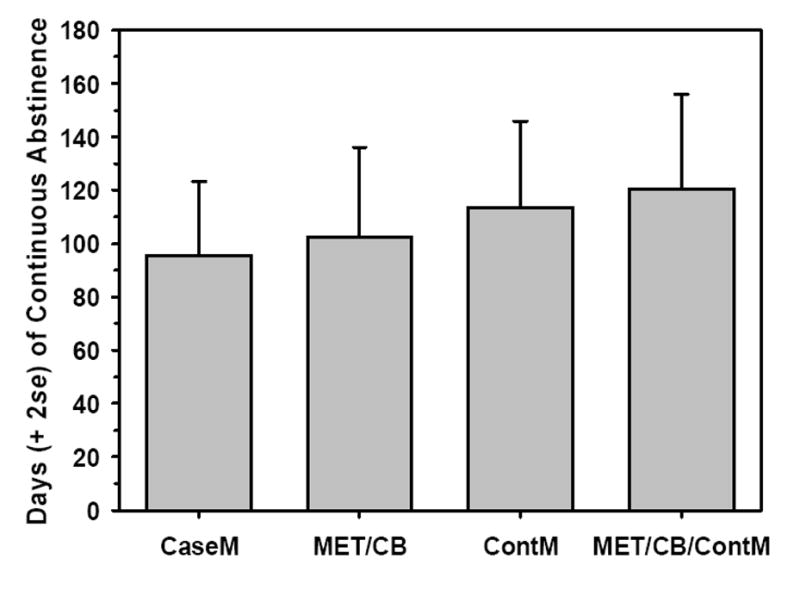
Longest period, in days, of continuous abstinence during the follow-up year, for each of the four interventions.
To determine whether there was a long-term change in drug use patterns, generalized estimating equations (GEE; Liang & Zeger, 1986) analyses were conducted to examine continuous abstinence in the time period prior to each follow-up point (Figure 4). This approach accounts for missing data by estimating the categorical outcome of the next event in a series based on the previous outcomes for a given subject. As with the mixed regression models used for the PDA outcome, the categorical outcome model was examined for effects attributable to Treatment, Time and Treatment X Time.
Figure 4.
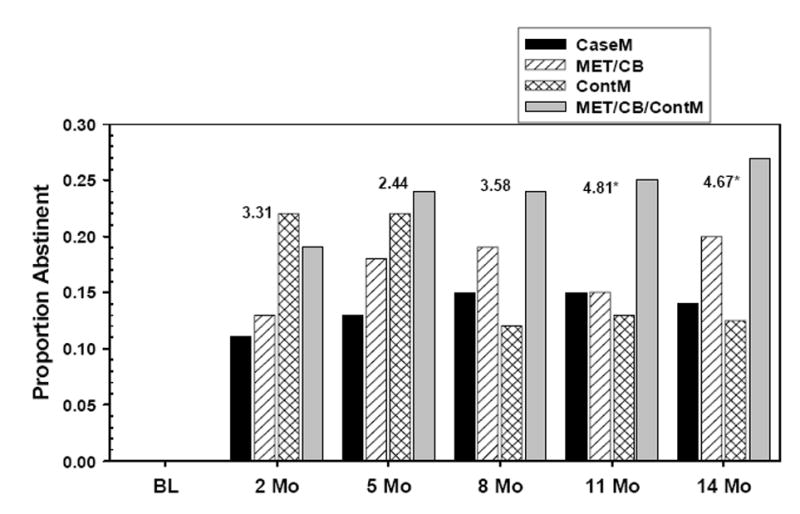
Proportion of clients who were abstinent throughout the measurement interval prior to each assessment point, for each intervention.
The chi-square values for each time point appear above each cluster of bars inFigure 4. Results from the GEE analyses showed a main effect for Treatment condition, with relatively high levels of continuous abstinence found for the two MET+CBT conditions, especially for MET+CBT+ContM at the later follow-ups (11 and 14 months). There was no Time effect, and no Treatment X Time interaction. At the final follow-up point, 14 months after entering the study, 27% of those in MET+CBT+ContM, and 19% in MET+CBT reported continuous abstinence in the previous 90 days (Figure 4). Despite good results for the ContM condition at posttreatment, continuous abstinence in that condition declined markedly after 5 months.
3.6 Time-to-Event Analyses
Cox regression analyses were conducted to examine the influence of treatment condition on time (in days) to the first use of marijuana. An event was defined as any self-reported smoking at any time after the baseline assessment session. PDA at baseline was entered first as a covariate, followed by the categorical Treatment condition variable. Planned contrasts like those used in the analysis of PDA were also examined. These analyses showed that 50% of the total sample smoked marijuana immediately after the baseline session, indicating that they never really stopped smoking. By the 14-month follow-up, approximately 18% reported never having relapsed over the entire 14-month period. The Cox regression analysis indicated no differential effect of Treatment on survival. Planned contrasts also did not yield any significant differences between Treatment conditions.
A second Cox regression analysis was conducted in which the event of interest was defined as the first episode of smoking after the first 30 days of treatment. This was done to allow an examination of survival rates once all patients had a chance to establish abstinence. This analysis, too, yielded no main effect for Treatment, although the planned contrast of ContM v. CaseM was significant (Wald χ2 = 4.54,p < .05; odds ratio = 0.56; CI: 0.33 to 0.95), indicating that those in the ContM condition were about half as likely to relapse after 30 days, relative to those treated in CaseM (seeFigure 5).
Figure 5.
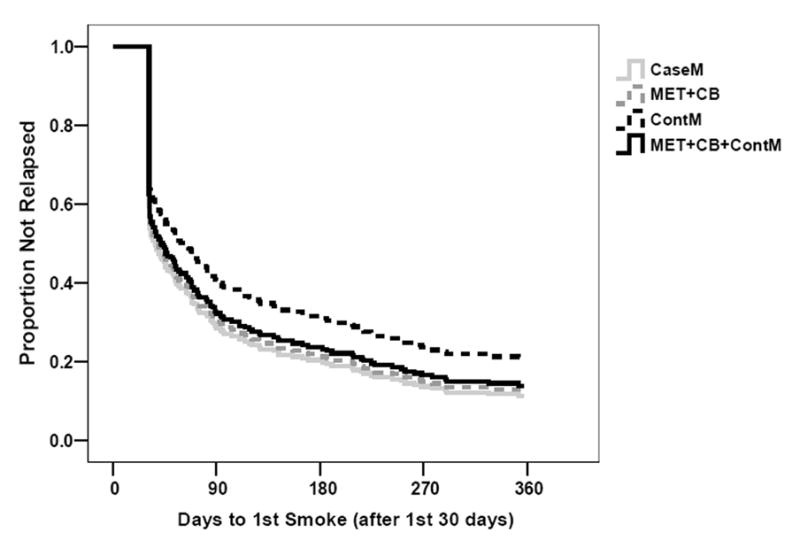
Survival curves for each intervention group, showing time to the first use of marijuana following the first 30 days of treatment.
3.7 Marijuana problems
Examination of mean problem scores over time indicated that the number of marijuana problems dropped significantly from baseline to posttreatment in all conditions, falling from a mean of 14 problems at baseline to a mean of fewer than 8 at posttreatment, and remaining at that level throughout the 14 month follow-up period. HLM analysis, like that used for the analysis of PDA, was used to evaluate the effect of treatment on marijuana-related problems. As before, Treatment was considered as a fixed effect, and the intercept a random effect. Based on RLL and AIC, an autoregressive covariance matrix (AR1) was adopted for this model. Results of this analysis yielded no main effect for treatment, a significant effect for Time [F (5, 214) = 41.95;p < .001], and no Treatment X Time interactions. Planned contrasts did not yield any difference in marijuana problem scores over time as a function of specific treatment comparisons.
3.8 Addiction Severity Index
Three composite subscales of the Addiction Severity Index (ASI) were also examined over time: the drug composite, the alcohol composite, and the psychiatric severity composite. These were administered at baseline, at posttreatment, and at the 8- and 14-month follow-ups. Mixed model regression analyses were conducted to evaluate the effect of treatment on the subscales over time. As before, Treatment was considered as a fixed effect, and the intercept a random effect. Based on RLL and AIC, an autoregressive covariance matrix was adopted for this model. Results were consistent across all three subscales: mean scores declined from pre- to post-treatment in all conditions. A significant Time effect emerged in all three analyses, but no main effect was seen for treatment, or for the treatment X time interaction.
3.9 Marijuana and Tobacco Use
Marijuana is often mixed with tobacco when smoked (e.g.,Patton et al. 2005), but the effects of tobacco smoking on cannabis use after treatment are not known. Analyses were repeated with level of tobacco smoking (average cigarettes smoked per day at baseline) used as a covariate to determine if tobacco smoking influenced treatment. Approximately 49% of the sample reported smoking cigarettes at baseline. This figure remained stable throughout the 14 months of the trial. Of those who did smoke, the majority smoked about 10 cigarettes per day. There were no differences in levels of cigarette smoking or proportions of cigarette smokers by treatment type for any time period. When used as a covariate in analyses of cannabis treatment response, level of cigarette smoking did not emerge as a significant contributor to outcome.
4. Discussion
It had been anticipated that the two ContM conditions, both of which provided tangible reinforcement for abstinence, would achieve the most abstinence, and would likely do so fairly early in treatment. It had also been anticipated that the group receiving MET+CBT in combination with ContM would be more likely to sustain abstinence during the follow-up period, after discontinuation of the reinforcement-for-abstinence contingency. These predictions were only partially fulfilled.
The ContM-only intervention, which reinforced marijuana-free urine specimens without the MET and CBT interventions, was associated with greatest PDA during treatment (as measured at posttreatment), and had the highest proportion of participants who had not yet relapsed at each follow-up assessment point. These findings partially support our prediction that ContM would have a substantial impact on initial abstinence. However, it is not clear why this superiority was limited to the ContM-only group, and not shared with the group that received MET+CBT in addition to ContM. There were some procedural differences between the two conditions, mainly that the ContM-only intervention was administered by Research Assistants who met with participants for about 15 minutes each week, whereas the ContM portion of the combined MET+CBT+ContM intervention was administered by the therapist who also provided the MET and CBT interventions in hour-long sessions. It seems unlikely that these procedural differences would have had such an impact; if anything, the differences should have favored the combined intervention.
The second prediction, that the group receiving MET+CBT+ContM combination would be the best at sustaining abstinence during the follow-up period, was partially realized. Although the abstinence rate for the combined MET+CBT+ContM group was not as high as the ContM-only group at posttreatment, the combined intervention was associated with the greatest number of participants who were abstinent late in the follow-up period and with the longest periods of continuous abstinence during the course of the follow-up year. These findings support our prediction that maintenance of abstinence over the course of the follow-up year would require the coping skills development offered by CBT.
Two other studies obtained similar patterns of results. In a study focused on treating cocaine use in methadone maintenance clients, Epstein, Hawkins, Covi, Umbricht, and Preston (2003)found that ContM rapidly reduced cocaine use, but the effect was dampened if ContM was augmented with CBT. At the 12-month follow-up, the initial benefits of ContM-alone had disappeared, but a reduction in cocaine use had emerged in the CBT+ContM group. Epstein et al. speculated that the late gains in the CBT+ContM group might have been due to a gradual process of learning that took place in the CBT component, an explanation that might also apply in the present case. They attributed the poorer initial effects of CBT+ContM to poorer attendance at CBT sessions, an explanation that does not apply to the present case where there were no differences in attendance across treatments.
In a second study with similar findings, Budney, Moore, Rocha, and Higgins (2006)found that ContM was significantly superior to CBT for promoting marijuana abstinence during treatment, and that the combination of CBT+ContM fell in between the other two, not significantly different from either of them. However, throughout the post-treatment follow-up year, CBT+ContM had the highest abstinence rates, leading the investigators to suggest that CBT helped maintain the initial effects of ContM.
The findings of the present study, as well as of Epstein et al. (2003)andBudney et al. (2006), are not consistent with a study of stimulant abusers. Rawson et al. (2006)found that ContM+CBT was as effective initially as ContM-alone; the effects lasted for a year, and CBT-alone produced comparable longer-term outcomes. Another inconsistency appears between the present study and the Budney et al. (2006)study in which a procedure akin to our MET+CBT+ContM condition was associated with a marijuana abstinence rate of 37% at the 12-month follow-up point, compared to 27% after 12 months in the present study. Factors that might account for these differences were 56% more sessions in the Budney et al. study, and the present study included 39% minorities whereas the Budney et al. study had about 4% minority participation; it is uncertain to what extent these factors may account for the differences in abstinence between the studies. Clearly, additional work is needed to sort out early and later effects, the mechanisms of action of ContM, CBT, and their combination, and perhaps also the impact of different populations of substance abusers and length of treatment.
The primary goal of this research had been to improve upon the abstinence rates observed in prior studies of treatments for marijuana dependence. In fact, the 27% one-year abstinence rate for the MET+CBT+ContM group was greater than any abstinence rate observed in the Marijuana Treatment Project (Marijuana Treatment Project Research Group, 2004), which did not employ a ContM intervention. Moore and Budney (2003)reported a similar marijuana abstinence rate of 29%, 6 months after various treatments, one of which included contingency management (data were not presented separately by treatment type, nor at 1 year). Both of these marijuana abstinence rates are somewhat less than the 34% rate reported at 7-month follow-up a in a study of relapse prevention for marijuana users, without a ContM intervention (Stephens et al., 2000). Speculations regarding the reasons for these differences include the relatively high socioeconomic and educational levels of participants, and the requirement of a $60 refundable deposit to participate in the Stephens et al. study.
In the present study, the highest abstinence rates were associated with one or the other of the ContM interventions, but they do not represent particularly high rates of abstinence. The effect of ContM on marijuana abstinence was modest, and the effect was limited to marijuana abstinence only, which was the target of ContM reinforcement. There were no differential effects of ContM on marijuana-related problems, or on drug use, alcohol use or psychiatric symptoms as assessed by the ASI.
The performance of the CaseM condition in this study was also notable. Although no skills relevant to controlling marijuana use were taught, and no systematic reinforcement for abstinence provided, participants in this condition achieved significant gains, about equal to those seen in the “active” conditions. Additional analysis will be needed to determine how CaseM achieved these outcomes.
The present study employed the same type of voucher-based reinforcement procedure that is commonly used in studies of ContM. However, the effect of reinforcement under this procedure is far from optimal because of the delay between the point at which a client successfully resists using marijuana and the time at which reinforcement is provided in the next treatment session. To be optimally beneficial, reinforcement should be provided almost immediately following occurrence of the desired behavior, i.e. at the time of each high-risk situation in which marijuana is not used. The delay of reinforcement problem is not unique to this study, but it may help to account for the weak effects of ContM that were observed here and in other studies as well.
Another potential limitation of the present study may be the magnitudes of reinforcement that were utilized. Most voucher-based studies in other drug-using populations have employed higher magnitudes ($1000 or more) than the $385 maximum in this study. Magnitude of reinforcement is known to be associated with outcomes in contingency management studies (Lussier et al., 2006). In the one study of ContM with marijuana users (Budney et al. 2000) that is similar to the present one, individual voucher values were less than in the present study (maximum of $46 in the final session), but urine specimens were collected more frequently (twice weekly) for a longer period of time (12 weeks), resulting in maximum total earnings of $570, which is $185 more than the maximum possible in the current study. Further work will be required to determine the impact of reinforcement frequency, quantity per opportunity, and total amount available, on treatment outcomes.
5. Summary
The ContM-only condition in the present study yielded the greatest PDA during treatment. However, the MET+CBT+ContM intervention was associated with the longest periods of continuous abstinence and the greatest number of participants abstinent towards the end of the follow-up year. The 27% one-year abstinence rate for the MET+CBT+ContM group was greater than that found in the Marijuana Treatment Project, but not as high as abstinence rates reported in other studies.
Despite the various advantages of the ContM interventions in the present study, ContM did not promote abstinence as much as had originally been anticipated. Other studies, by our research group and other investigators, have identified client variables, notably self-efficacy, that may be mediators of treatment success (e.g., Litt, Kadden, Cooney & Kabela, 2003;Litt et al., 2005). Analyses of the mechanisms of treatment will be necessary to fully understand the roles of CBT and contingency management in the long-term outcomes of marijuana-dependent patients.
Acknowledgments
Support for this project was provided by grant R01-DA12728 from the National Institute on Drug Abuse, and in part by General Clinical Research Center grant M01-RR06192 from the National Institutes of Health. The authors would like to acknowledge the therapists: Aimee Markward, MS, CDAC, Susan Sampl, PhD, and Jay Beatman, PsyD; and the Research Assistants: Priscilla Morse, Kara Dion, and Abigail Sama.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allsop S, Saunders B, Phillips M, Carr A. A trial of relapse prevention with severely dependent male problem drinkers. Addiction. 1997;92:61–74. [PubMed] [Google Scholar]
- Bell R, Wechsler H, Johnston LD. Correlates of college student marijuana use: Results of a US National Survey. Addiction. 1997;92:571–581. [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical linear models: Applications and data analysis methods. Newbury Park, CA: Sage; 1992. [Google Scholar]
- Budney AJ, Higgins ST. National Institute on Drug Abuse Publication Number 98-4309. Rockville, MD: National Institute on Drug Abuse; 1998. A community reinforcement plus vouchers approach: Treating cocaine addiction. [Google Scholar]
- Budney AJ, Higgins ST, Delaney DD, Kent L, Bickel WK. Contingent reinforcement of abstinence with individuals abusing cocaine and marijuana. Journal of Applied Behavior Analysis. 1991;24:657–665. doi: 10.1901/jaba.1991.24-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. Journal of Consulting and Clinical Psychology. 2000;68:1051–1061. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. Journal of Consulting and Clinical Psychology. 2006;74:307–316. doi: 10.1037/0022-006X.4.2.307. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- DeRubeis RJ, Crits-Christoph P. Empirically supported individual and group psychological treatments for adult mental disorders. Journal of Consulting and Clinical Psychology. 1998;66:37–52. doi: 10.1037//0022-006x.66.1.37. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Hawkins WE, Covi L, Umbricht A, Preston KL. Cognitive-behavioral therapy plus contingency management for cocaine use: Findings during treatment and across 12-month follow-up. Psychology of Addictive Behaviors. 2003;17:73–82. doi: 10.1037/0893-164X.17.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders – Patient edition (SCID-I/P, Version 20) Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Griffiths RR, Bigelow GE, Liebson I. Relationship of social factors to ethanol self-administration in alcoholics. In: Nathan PE, Marlatt GA, Loberg T, editors. Alcoholism: New directions in behavioral research and treatment. New York: Plenum Press; 1978. pp. 351–379. [Google Scholar]
- Higgins ST, Alessi SM, Dantona RL. Voucher-based incentives: A substance abuse treatment innovation. Addictive Behaviors. 2002;27:887–910. doi: 10.1016/s0306-4603(02)00297-6. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Archives of General Psychiatry. 1994;51:568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Litt MD, Kadden RM, Cooney NL, Kabela E. Coping skills and treatment outcomes in cognitive-behavioral and interactional group therapy for alcoholism. Journal of Consulting and Clinical Psychology. 2003;71:118–128. doi: 10.1037//0022-006x.71.1.118. [DOI] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, Stephens R, the Marijuana Treatment Project Research Group Coping and self-efficacy in marijuana treatment: Results from the Marijuana Treatment Project. Journal of Consulting and Clinical Psychology. 2005;73:1015–1025. doi: 10.1037/0022-006X.73.6.1015. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. New York: Wiley; 1987. [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Marijuana Treatment Project Research Group Brief treatments for cannabis dependence: Findings from a randomized multisite trial. Journal of Consulting and Clinical Psychology. 2004;72:455–466. doi: 10.1037/0022-006X.72.3.455. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Grisson GR, Zanis D, Randall M, Brill P, O’Brien C. Problem-service ‘matching’ in addiction treatment. Archives of General Psychiatry. 1997;54:730–735. doi: 10.1001/archpsyc.1997.01830200062008. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O’Brien CP. New data from the Addiction Severity Index. Reliability and validity in three centers. The Journal of Nervous and Mental Disease. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- McRae AL, Budney AJ, Brady KT. Treatment of marijuana dependence: A review of the literature. Journal of Substance Abuse Treatment. 2003;24:369–376. doi: 10.1016/s0740-5472(03)00041-2. [DOI] [PubMed] [Google Scholar]
- Miller WR, Brown JM, Simpson TL, et al. What works? A methodological analysis of the alcohol treatment outcome literature. In: Hester RK, Miller WR, editors. Handbook of alcoholism treatment approaches: Effective alternatives. 2. New Jersey: Allyn and Bacon; 1995. pp. 12–44. [Google Scholar]
- Miller WR, Zweben A, DiClemente CC, Rychtarik RG. Motivational enhancement therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence, Vol. 2, Project MATCH Monograph Series. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1992. [Google Scholar]
- Monti PM, Abrams DB, Kadden RM, Cooney NL. Treating alcohol dependence: A coping skills training guide. New York: Guilford Press; 1989. [Google Scholar]
- Monti PM, Kadden RM, Rohsenow DJ, Cooney NL, Abrams DB. Treating alcohol dependence: A coping skills training guide. 2. New York: Guilford Press; 2002. [Google Scholar]
- Patton GC, Coffey C, Carlin JB, Sawyer SM, Lynskey M. Reverse gateways? Frequent cannabis use as a predictor of tobacco initiation and nicotine dependence. Addiction. 2005;100:1518–1525. doi: 10.1111/j.1360-0443.2005.01220.x. [DOI] [PubMed] [Google Scholar]
- Moore BA, Budney AJ. Relapse in outpatient treatment for marijuana dependence. Journal of Substance Abuse Treatment. 2003;25:85–89. doi: 10.1016/s0740-5472(03)00083-7. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Tedford J, Austin M, Tardif M. Vouchers versus prizes: Contingency management for treatment of substance abusers in community settings. Journal of Consulting and Clinical Psychology. 2005;73:1005–1114. doi: 10.1037/0022-006X.73.6.1005. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B. Prize reinforcement contingency management for cocaine dependence: Integration with group therapy in a methadone clinic. Journal of Consulting and Clinical Psychology. 2005;73:354–359. doi: 10.1037/0022-006X.73.2.354. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes, and they will come: Contingency management for treatment of alcohol dependence. Journal of Consulting and Clinical Psychology. 2000;68:250–257. doi: 10.1037//0022-006x.68.2.250. [DOI] [PubMed] [Google Scholar]
- Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs. Archives of General Psychiatry. 2005;62:1148–1156. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- Petry NM, Tedford J, Austin M, Nich C, Carroll KM, Rounsaville BJ. Prize reinforcement contingency management for treatment of cocaine abusers: How low can we go, and with whom? Addiction. 2004;99:349–360. doi: 10.1111/j.1360-0443.2003.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C, et al. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101:267–274. doi: 10.1111/j.1360-0443.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- Roffman RA, Klepsch R, Wertz JS, Simpson EE, Stephens RS. Predictors of attrition from an outpatient marijuana-dependence counseling program. Addictive Behaviors. 1993;18:553–566. doi: 10.1016/0306-4603(93)90071-g. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Shoptaw S, Jarvik ME, Ling W, Rawson RA. Contingency management of tobacco smoking in methadone-maintained opiate addicts. Addictive Behaviors. 1996;21:409–412. doi: 10.1016/0306-4603(95)00066-6. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. pp. 41–71. [Google Scholar]
- Sobell MB, Maisto SA, Sobell LC, Cooper AM, Cooper T, Sanders BLC. Developing a prototype for evaluating alcohol treatment effectiveness. In: Sobell MB, Sobel, Ward E, editors. Evaluating alcohol and drug abuse treatment effectiveness: Recent advances. New York: Pergamon; 1980. [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. Journal of the American Medical Association. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA. Treating adult marijuana dependence. 1996 doi: 10.1037//0022-006x.62.1.92. Paper presented at the 58th annual scientific meeting of the College on Problems of Drug Dependence San Juan, Puerto Rico. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. Journal of Consulting and Clinical Psychology. 2000;68:898–908. [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Simpson EE. Treating adult marijuana dependence: A test of the relapse prevention model. Journal of Consulting & Clinical Psychology. 1994;62:92–99. doi: 10.1037//0022-006x.62.1.92. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Wertz JS, Roffman RA. Predictors of marijuana treatment outcomes: The role of self-efficacy. Journal of Substance Abuse. 1993;5:341–353. doi: 10.1016/0899-3289(93)90003-t. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE, Liebson IA, Hawthorne JW. Contingent reinforcement of benzodiazepine-free urines: Evaluation of a drug abuse treatment intervention. Journal of Applied Behavior Analysis. 1982;15:493–503. doi: 10.1901/jaba.1982.15-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. Alcoholism treatment matching research: Methodological and clinical approaches. In: Donovan DM, Mattson ME, editors. Journal of Studies on Alcohol. Supplement 12. 1994. pp. 70–75. [DOI] [PubMed] [Google Scholar]
- Troisi A, Pasini A, Saracco M, Spalletta G. Psychiatric symptoms in male cannabis users not using other illicit drugs. Addiction. 1998;93:487–492. doi: 10.1046/j.1360-0443.1998.9344874.x. [DOI] [PubMed] [Google Scholar]


