Abstract
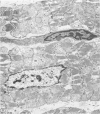
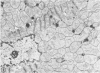
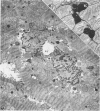
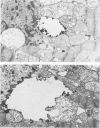
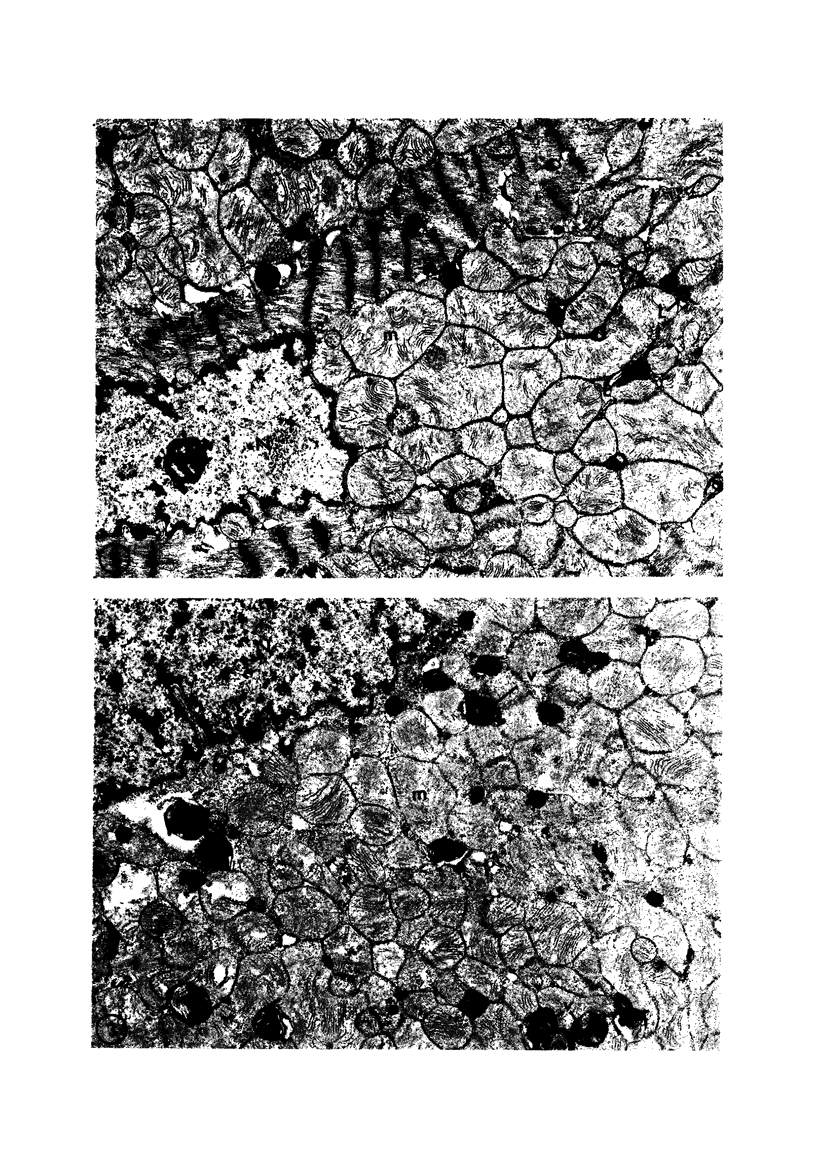
Rabbit hearts perfused under hypoxic conditions underwent progressive subcellular damage, which becomes irreversible by one hour. During the first 20 minutes of perfusion, minor dilation of mitochondria and condensation of nuclear chromatin were the only salient features of cell injury. By 40 minutes moderate mitochondrial swelling was evident in hypoxic myocytes. Moreover, an increase in degenerating mitochondria and autophagic vacuoles was apparent. Reperfusion after either 20 or 40 minutes of hypoxia restored contractility, and injured myocytes underwent a cellular repair process that involved a dramatic increase in lysosomal autoplagy. One hour of hypoxia yielded irreversibly injured myocytes. Upon reoxygenation, some of these cells displayed typical changes of necrosis, but others apparently underwent an abortive repair process involving the formation of large, probably nonfunctional lysosomes. These observations suggest that lysosomal autophagy is important in the efforts at repair that cardiac cells initiate during and after hypoxia.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunk U. T., Ericsson J. L. Cytochemical evidence for the leakage of acid phosphatase through ultrastructurally intact lysosomal membranes. Histochem J. 1972 Nov;4(6):479–491. doi: 10.1007/BF01011128. [DOI] [PubMed] [Google Scholar]
- Decher R. S., Poole A. R., Dingle J. T., Wildenthal K. Influence of methylprednisolone of the sequential redistribution of cathepsin D and other lysosomal enzymes during myocardial ischemia in rabbits. J Clin Invest. 1978 Oct;62(4):797–804. doi: 10.1172/JCI109191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker R. S., Poole A. R., Crie J. S., Dingle J. T., Wildenthal K. Lysosomal alterations in hypoxic and reoxygenated hearts. II. Immunohistochemical and biochemical changes in cathepsin D. Am J Pathol. 1980 Feb;98(2):445–456. [PMC free article] [PubMed] [Google Scholar]
- Decker R. S., Poole A. R., Dingle J. T., Wildenthal K. Lysosomal alterations in autolyzing rabbit heart. J Mol Cell Cardiol. 1979 Feb;11(2):189–196. doi: 10.1016/0022-2828(79)90463-2. [DOI] [PubMed] [Google Scholar]
- Decker R. S., Poole A. R., Griffin E. E., Dingle J. T., Wildenthal K. Altered distribution of lysosomal cathepsin D in ischemic myocardium. J Clin Invest. 1977 May;59(5):911–921. doi: 10.1172/JCI108713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker R. S., Wildenthal K. Influence of methylprednisolone on ultrastructural and cytochemical changes during myocardial ischemia. Selective effects on various cell inclusions and organelles including lysosomes. Am J Pathol. 1978 Jul;92(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- Decker R. S., Wildenthal K. Sequential lysosomal alterations during cardiac ischemia. II. Ultrastructural and cytochemical changes. Lab Invest. 1978 Jun;38(6):662–673. [PubMed] [Google Scholar]
- Ganote C. E., Seabra-Gomes R., Nayler W. G., Jennings R. B. Irreversible myocardial injury in anoxic perfused rat hearts. Am J Pathol. 1975 Sep;80(3):419–450. [PMC free article] [PubMed] [Google Scholar]
- Goldfischer S. The cytochemical demonstration of lysosomal aryl sulfatase activity by light and electron microscopy. J Histochem Cytochem. 1965 Jul-Aug;13(6):520–523. doi: 10.1177/13.6.520. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Humphrey S. M., Chain E. B. Abrupt reoxygenation of the anoxic potassium-arrested perfused rat heart: a study of myocardial enzyme release. J Mol Cell Cardiol. 1973 Aug;5(4):395–407. doi: 10.1016/0022-2828(73)90030-8. [DOI] [PubMed] [Google Scholar]
- Hoffstein S., Gennaro D. E., Weissmann G., Hirsch J., Streuli F., Fox A. C. Cytochemical localization of lysosomal enzyme activity in normal and ischemic dog myocardium. Am J Pathol. 1975 May;79(2):193–206. [PMC free article] [PubMed] [Google Scholar]
- Hopsu-Havu V. K., Arstila A. U., Helminen H. J., Kalimo H. O. Improvements in the method for the electron microscopic localization of arylsulphatase activity. Histochemie. 1967;8(1):54–64. doi: 10.1007/BF00279874. [DOI] [PubMed] [Google Scholar]
- Ingwall J. S., DeLuca M., Sybers H. D., Wildenthal K. Fetal mouse hearts: a model for studying ischemia. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2809–2813. doi: 10.1073/pnas.72.7.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings R. B., Ganote C. E. Structural changes in myocardium during acute ischemia. Circ Res. 1974 Sep;35 (Suppl 3):156–172. [PubMed] [Google Scholar]
- Kloner R. A., Ganote C. E., Whalen D. A., Jr, Jennings R. B. Effect of a transient period of ischemia on myocardial cells. II. Fine structure during the first few minutes of reflow. Am J Pathol. 1974 Mar;74(3):399–422. [PMC free article] [PubMed] [Google Scholar]
- MORGAN H. E., HENDERSON M. J., REGEN D. M., PARK C. R. Regulation of glucose uptake in muscle. I. The effects of insulin and anoxia on glucose transport and phosphorylation in the isolated, perfused heart of normal rats. J Biol Chem. 1961 Feb;236:253–261. [PubMed] [Google Scholar]
- McCallister L. P., Munger B. L., Neely J. R. Electron microscopic observations and acid phosphatase activity in the ischemic rat heart. J Mol Cell Cardiol. 1977 May;9(5):353–364. doi: 10.1016/s0022-2828(77)80002-3. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Rovetto M. J., Whitmer J. T., Morgan H. E. Effects of ischemia on function and metabolism of the isolated working rat heart. Am J Physiol. 1973 Sep;225(3):651–658. doi: 10.1152/ajplegacy.1973.225.3.651. [DOI] [PubMed] [Google Scholar]
- Wildenthal K., Decker R. S., Poole A. R., Griffin E. E., Dingle J. T. Sequential lysosomal alterations during cardiac ischemia. I. Biochemical and immunohistochemical changes. Lab Invest. 1978 Jun;38(6):656–661. [PubMed] [Google Scholar]
- Wildenthal K. Lysosomal alterations in ischemic myocardium: result or cause of myocellular damage? J Mol Cell Cardiol. 1978 Jul;10(7):595–603. doi: 10.1016/s0022-2828(78)80001-7. [DOI] [PubMed] [Google Scholar]