Abstract
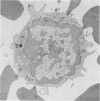
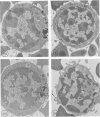
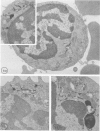
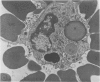
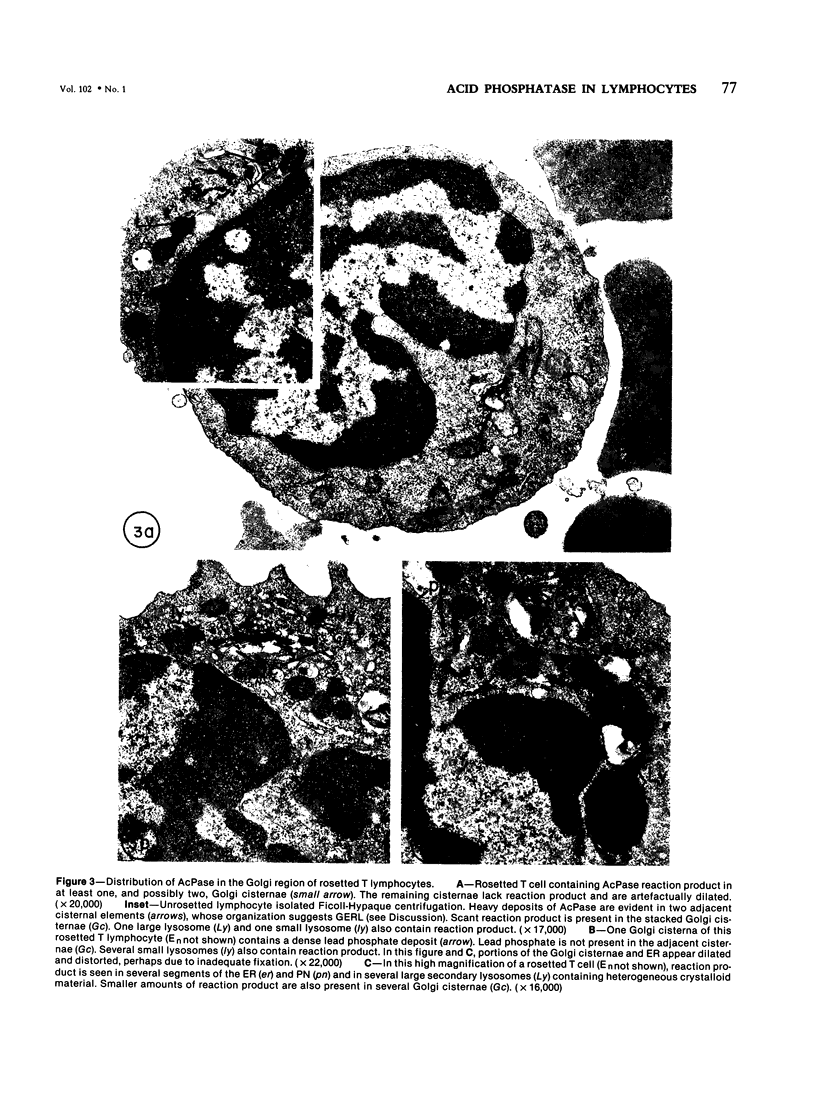
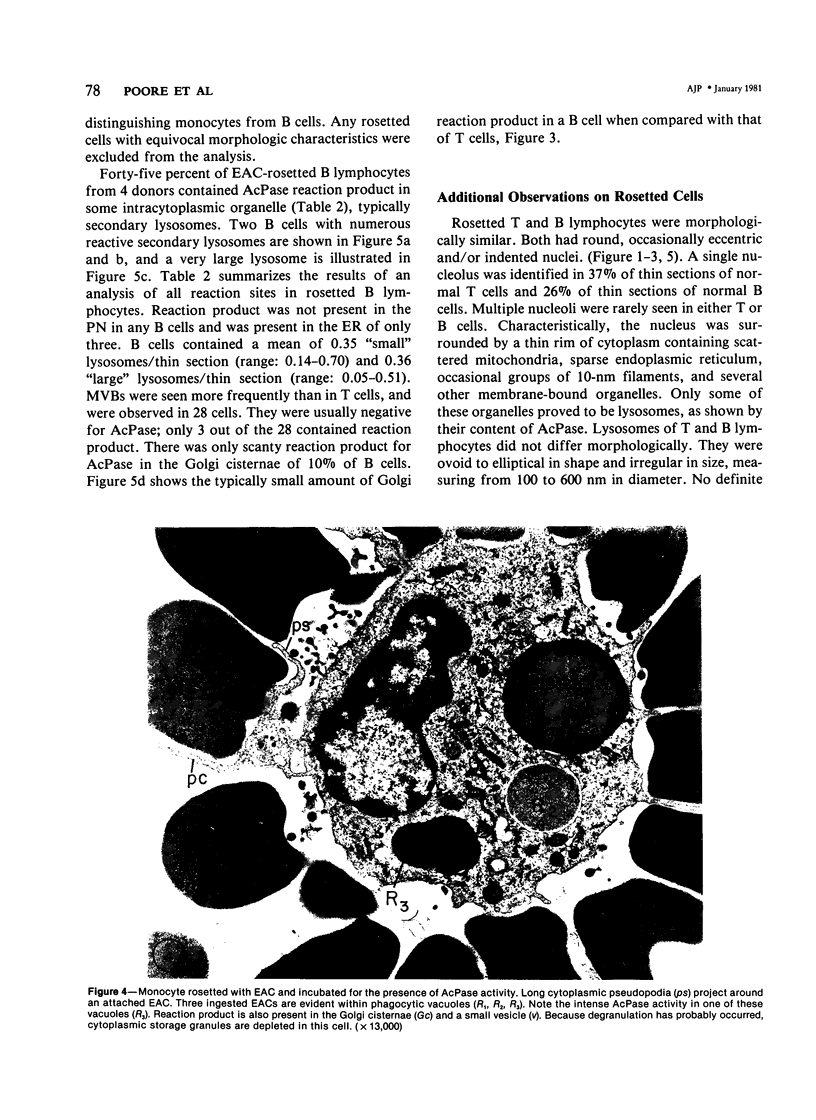
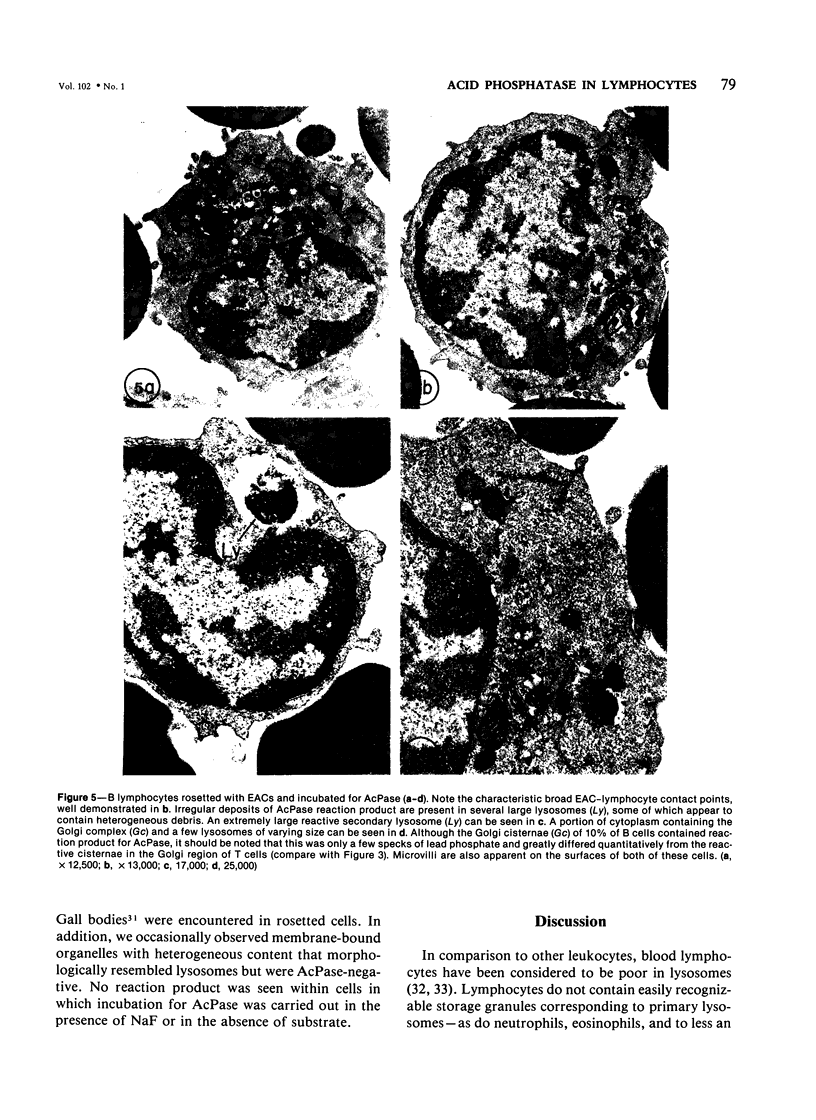
Using electron microscopy and cytochemical techniques, the authors determined the distribution of acid phosphatase (AcPase) within the organelles of lymphocytes from blood rosetted with either neuraminidase-treated sheep erythrocytes (En) or sheep erythrocytes coated with antibody and complement (EACs). Subsequently, the various reactive organelles of the rosetted lymphocytes were counted, affording a comparison of T and B cells. It was found that AcPase was present in approximately 80% of T cells and 45% of B cells and was most frequently observed in secondary lysosomes of varying size and content. Although more T cells than B cells were reactive for AcPase, the extent of reaction in some B cells clearly precludes the use of AcPase for differentiating the two cell lines. It should be recognized that while the En rosetting procedure detects T cells in a nonselective manner, the EAC rosette is a marker of a major subpopulation of B lymphocytes, ie, those bearing complement receptors. We believe that the distribution of lysosomal enzymes in B and T lymphocytes probably reflects the functional state of individual cells rather than being a reliable indicator of cell lineage. A surprising finding (which could be established only by a fine-structural study) was the fact that 20% of circulating "resting" T cells contained reaction product for AcPase within endoplasmic reticulum and the perinuclear cisterna indicating that these cells are actively synthesizing AcPase, probably due to a foregoing inductive event. Such stimulus could be the result of recent endocytosis of surface receptors in combination with antigen, antibody, or immune complexes and/or recent mitosis, or possibly some unrelated autophagic incident.
Full text
PDF
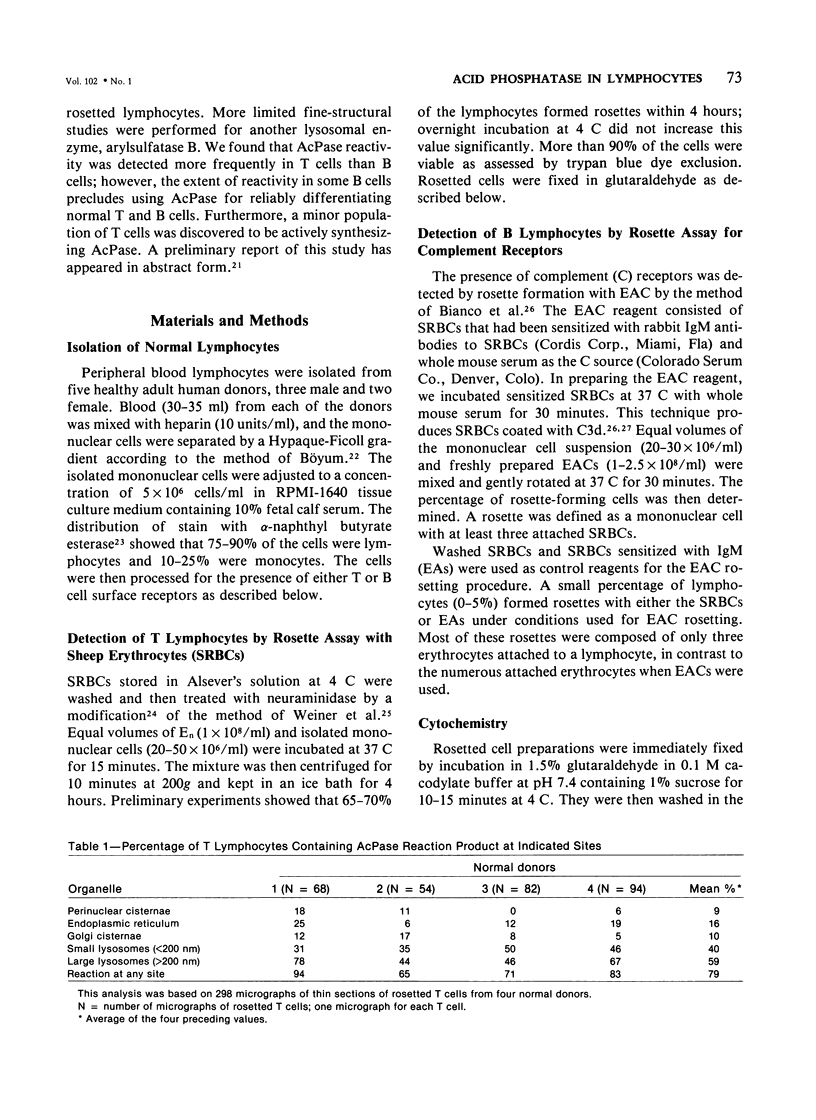

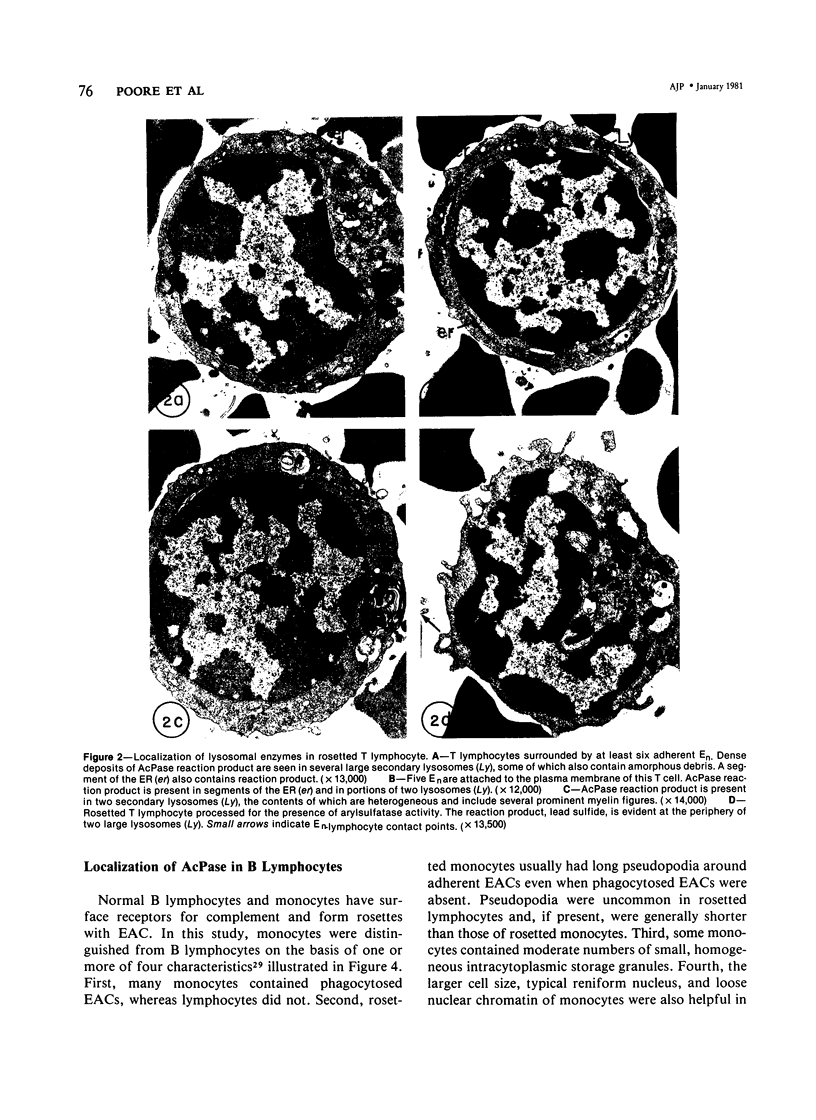




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. C., Gahmberg C. G. Surface glycoproteins of resting and activated human T lymphocytes. Mol Cell Biochem. 1979 Oct 15;27(2):117–131. doi: 10.1007/BF00218355. [DOI] [PubMed] [Google Scholar]
- Axline S. G., Cohn Z. A. In vitro induction of lysosomal enzymes by phagocytosis. J Exp Med. 1970 Jun 1;131(6):1239–1260. doi: 10.1084/jem.131.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr R. D., Perry S. Lysosomal acid hydrolases in human lymphocyte subpopulations. Br J Haematol. 1976 Apr;32(4):565–572. doi: 10.1111/j.1365-2141.1976.tb00960.x. [DOI] [PubMed] [Google Scholar]
- Barrett S. Tissue distribution of human T cells with complement receptors (D cells). Clin Immunol Immunopathol. 1978 Oct;11(2):190–195. doi: 10.1016/0090-1229(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Bentwich Z., Douglas S. D., Siegal F. P., Kunkel H. G. Human lymphocyte-sheep erythrocyte rosette formation: some characteristics of the interaction. Clin Immunol Immunopathol. 1973 Jul;1(4):511–522. doi: 10.1016/0090-1229(73)90007-x. [DOI] [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biberfeld P. Endocytosis and lysosome formation in blood lymphocytes transformed by phytohemagglutinin. J Ultrastruct Res. 1971 Oct;37(1):41–68. doi: 10.1016/s0022-5320(71)80040-0. [DOI] [PubMed] [Google Scholar]
- Brittinger G., Hirschhorn R., Douglas S. D., Weissmann G. Studies on lysosomes. XI. Characterization of a hydrolase-rich fraction from human lymphocytes. J Cell Biol. 1968 May;37(2):394–411. doi: 10.1083/jcb.37.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouet J. C., Sasportes M., Flandrin G., Preud'Homme J. L., Seligmann M. Chronic lymphocytic leukaemia of T-cell origin. Immunological and clinical evaluation in eleven patients. Lancet. 1975 Nov 8;2(7941):890–893. doi: 10.1016/s0140-6736(75)92127-3. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of leucocytes from human blood. A two-phase system for removal of red cells with methylcellulose as erythrocyte-aggregating agent. Scand J Clin Lab Invest Suppl. 1968;97:9–29. [PubMed] [Google Scholar]
- Catovsky D. Letter: T-cell origin of acid-phosphatase-positive lymphoblasts. Lancet. 1975 Aug 16;2(7929):327–328. doi: 10.1016/s0140-6736(75)92767-1. [DOI] [PubMed] [Google Scholar]
- Douglas S. D., Cohnen G., König E., Brittinger G. Lymphocyte lysosomes and lysosomal enzymes in chronic lymphocytic leukemia. Blood. 1973 Apr;41(4):511–518. [PubMed] [Google Scholar]
- Ehlenberger A. G., McWilliams M., Phillips-Quagliata J. M., Lamm M. E., Nussenzweig V. Immunoglobulin-bearing and complement-receptor lymphocytes constitute the same population in human peripheral blood. J Clin Invest. 1976 Jan;57(1):53–56. doi: 10.1172/JCI108268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandrin G., Brouet J. C. The Sezary cell: cytologic, cytochemical, and immunologic studies. Mayo Clin Proc. 1974 Aug;49(8):575–583. [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K., Gonatas J. O., Stieber A., Antoine J. C., Avrameas S. Quantitative ultrastructural autoradiographic studies of iodinated plasma membranes of lymphocytes during segregation and internalization of surface immunoglobulins. J Cell Biol. 1976 Sep;70(3):477–493. doi: 10.1083/jcb.70.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R., Brittinger G., Hirschhorn K., Weissmann G. Studies on lysosomes. XII. Redistribution of acid hydrolases in human lymphocytes stimulated by phytohemagglutinin. J Cell Biol. 1968 May;37(2):412–423. doi: 10.1083/jcb.37.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R., Hirschhorn K., Weissmann G. Appearance of hydrolase rich granules in human lymphocytes induced by phytohemagglutinin and antigens. Blood. 1967 Jul;30(1):84–102. [PubMed] [Google Scholar]
- Howard J. C. The life-span and recirculation of marrow-derived small lymphocytes from the rat thoracic duct. J Exp Med. 1972 Feb 1;135(2):185–199. doi: 10.1084/jem.135.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling R., Wigzell H. An analysis of the murine NK cell as to structure, function and biological relevance. Immunol Rev. 1979;44:165–208. doi: 10.1111/j.1600-065x.1979.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Li C. Y., Lam K. W., Yam L. T. Esterases in human leukocytes. J Histochem Cytochem. 1973 Jan;21(1):1–12. doi: 10.1177/21.1.1. [DOI] [PubMed] [Google Scholar]
- Linthicum D. S., Elson E., Mendelsohn J., Sell S. Endocytosis and exocytosis of phytohemagglutinin (PHA) cell surface receptors of human lymphocytes during blast transformation. Exp Cell Res. 1977 Dec;110(2):237–250. doi: 10.1016/0014-4827(77)90289-0. [DOI] [PubMed] [Google Scholar]
- Mackiewicz S., Brelińska-Peczalska R., Celińska-Szpytko E. Cytochemical studies of rosette-forming cells. Z Immunitatsforsch Exp Klin Immunol. 1975 May;148(5):462–466. [PubMed] [Google Scholar]
- Meusers P., König E., Fink U., Brittinger G. Lysosomal acid phosphatase: difference between normal and chronic lymphocytic leukaemia T and B lymphocytes. Blut. 1976 Nov;33(5):313–318. doi: 10.1007/BF01002128. [DOI] [PubMed] [Google Scholar]
- Mueller J., Brun del Re G., Buerki H., Keller H. U., Hess M. W., Cottier H. Nonspecific acid esterase activity: a criterion for differentiation of T and B lymphocytes in mouse lymph nodes. Eur J Immunol. 1975 Apr;5(4):270–274. doi: 10.1002/eji.1830050411. [DOI] [PubMed] [Google Scholar]
- Nichols B. A., Bainton D. F. Differentiation of human monocytes in bone marrow and blood. Sequential formation of two granule populations. Lab Invest. 1973 Jul;29(1):27–40. [PubMed] [Google Scholar]
- Novikoff A. B. The endoplasmic reticulum: a cytochemist's view (a review). Proc Natl Acad Sci U S A. 1976 Aug;73(8):2781–2787. doi: 10.1073/pnas.73.8.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangalis G. A., Kuhl W., Waldman S. R., Beutler E. Acid hydrolases in normal B and T blood lymphocytes. Acta Haematol. 1978;59(5):285–292. doi: 10.1159/000207774. [DOI] [PubMed] [Google Scholar]
- Pangalis G. A., Waldman S. R., Rappaport H. Cytochemical findings in human nonneoplastic blood and tonsillar B and T lymphocytes. Am J Clin Pathol. 1978 Mar;69(3):314–318. doi: 10.1093/ajcp/69.1.314. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Cell-surface immunology. Sci Am. 1976 May;234(5):30–39. doi: 10.1038/scientificamerican0576-30. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Nadler L. M., Sallan S. E., Schlossman S. F. Subset derivation of T-cell acute lymphoblastic leukemia in man. J Clin Invest. 1979 Aug;64(2):392–397. doi: 10.1172/JCI109474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter J., Gaedicke G., Winkler K., Beckmann H., Landbeck G. Letter: Possible T-cell origin of lymphoblasts in acid-phosphatase-positive acute lymphatic leukaemia. Lancet. 1975 Jul 12;2(7924):75–75. doi: 10.1016/s0140-6736(75)90515-2. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. S., Davie J. M., Rosenstreich D. L., Cehrs K. U. Antibody-mediated internalization of B lymphocyte surface membrane immunoglobulin. Exp Cell Res. 1973 Oct;81(2):317–329. doi: 10.1016/0014-4827(73)90521-1. [DOI] [PubMed] [Google Scholar]
- Ross G. D. Identification of human lymphocyte subpopulations by surface marker analysis. Blood. 1979 May;53(5):799–811. [PubMed] [Google Scholar]
- Unanue E. R. Cellular events folowing binding of antigen to lymphocytes. Am J Pathol. 1974 Oct;77(1):2–22. [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Perkins W. D., Karnovsky M. J. Ligand-induced movement of lymphocyte membrane macromolecules. I. Analysis by immunofluorescence and ultrastructural radioautography. J Exp Med. 1972 Oct 1;136(4):885–906. doi: 10.1084/jem.136.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSMANN G. LYSOSOMES. Blood. 1964 Nov;24:594–606. [PubMed] [Google Scholar]
- Wehinger H., Möbius W. Cytochemical studies on T and B lymphocytes and lymphoblasts with special reference to acid phosphatase. Acta Haematol. 1976;56(3):129–136. doi: 10.1159/000207929. [DOI] [PubMed] [Google Scholar]
- Weiner M. S., Bianco C., Nussenzweig V. Enhanced binding of neuraminidase-treated sheep erythrocytes to human T lymphocytes. Blood. 1973 Dec;42(6):939–946. [PubMed] [Google Scholar]