Abstract
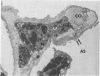
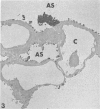
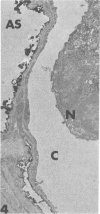
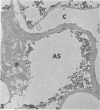
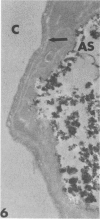
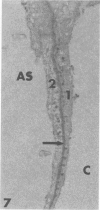
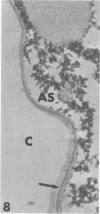
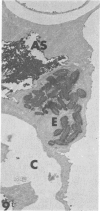
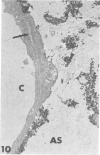
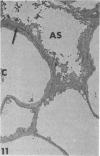
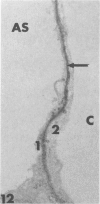
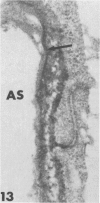
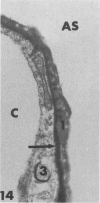
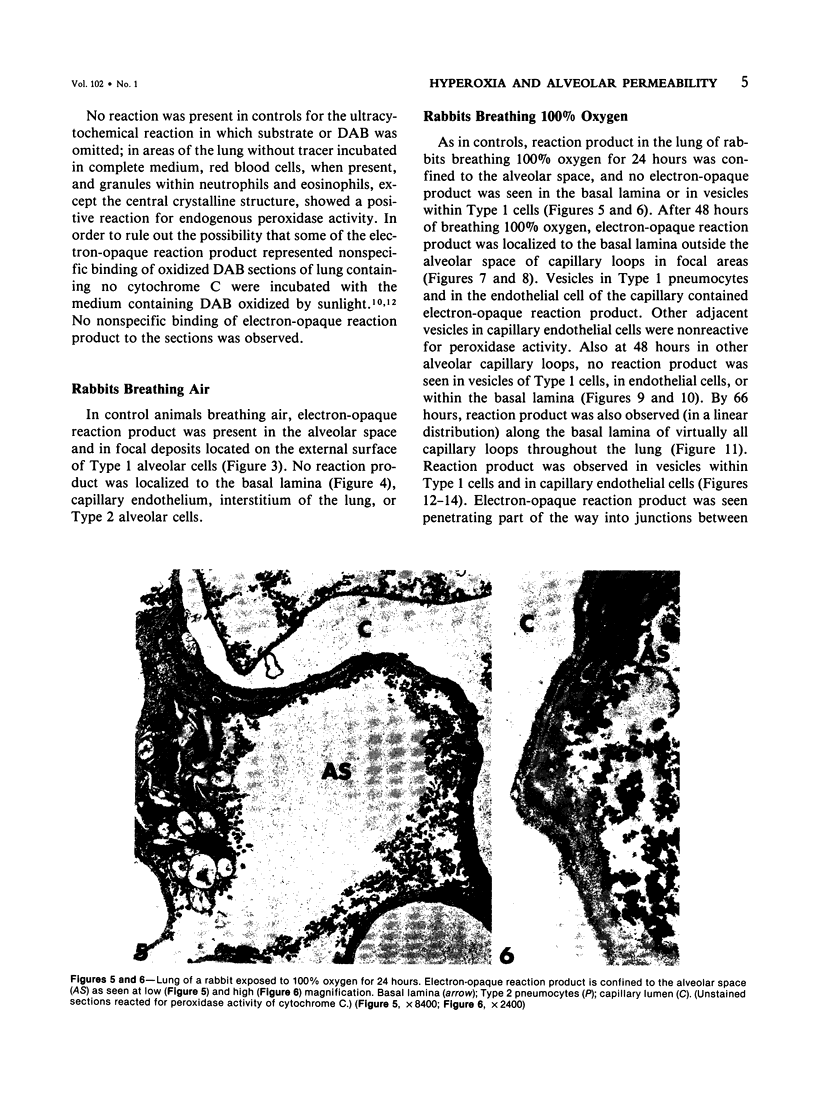
Rabbits were exposed to 100% oxygen or to air at one atmosphere. No alterations were observed in the lung of rabbits breathing air for up to 66 hours or 100% oxygen for 24 hours; after 48 hours, inflammatory cells, chiefly neutrophils, were located in the interstitium of the lung. By 66 hours of oxygen, the number of inflammatory cells in the interstitial space was greater than at 48 hours. At 72 hours, alveolar space in focal areas of the lung was filled with edema fluid containing a lightly flocculent material, and more densely staining fibrin. In experiments for the study of alveolar permeability, cytochrome C was instilled through the tracheobronchial tree into alveoli and demonstrated ultracytochemically by its peroxidase activity. No electron-opaque reaction product was observed in control rabbits or in those breathing oxygen for 24 hours, indicating that the tracer did not leave the alveolar space. However, after 48 hours of the breathing of 100% oxygen, electron-opaque reaction product was localized to the basal lamina of alveolar capillaries in focal areas, whereas in other alveolar capillaries there was no reaction product in the basal lamina. Vesicles filled with reaction product were observed in Type 1 pneumocytes and in alveolar capillary endothelial cells within capillary loops having increased electron density in the basal lamina. After 66 hours of the breathing of 100% oxygen, virtually all alveolar capillaries showed electron-opaque reaction product in the basal lamina and in vesicles within Type 1 cells and capillary endothelial cells. Increased permeability of Type 1 pneumocytes appears as an early manifestation of oxygen-induced changes in the lung preceding pulmonary edema. The presence of numerous inflammatory cells in the interstitium and in alveolar capillaries may play some part in the pathogenesis of the oxygen-induced increase in alveolar permeability.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chinard F. P. The alveolar-capillary barrier: some data and speculations. Microvasc Res. 1980 Jan;19(1):1–17. doi: 10.1016/0026-2862(80)90081-3. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Lambertsen C. J. Pulmonary oxygen toxicity: a review. Pharmacol Rev. 1971 Jun;23(2):37–133. [PubMed] [Google Scholar]
- Gil J. Ultrastructure of lung fixed under physiologically defined conditions. Arch Intern Med. 1971 May;127(5):896–902. [PubMed] [Google Scholar]
- Gorin A. B., Stewart P. A. Differential permeability of endothelial and epithelial barriers to albumin flux. J Appl Physiol Respir Environ Exerc Physiol. 1979 Dec;47(6):1315–1324. doi: 10.1152/jappl.1979.47.6.1315. [DOI] [PubMed] [Google Scholar]
- Hand W. L., Cantey J. R. Antibacterial mechanisms of the lower respiratory tract. I. Immunoglobulin synthesis and secretion. J Clin Invest. 1974 Feb;53(2):354–362. doi: 10.1172/JCI107567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugaard N. Cellular mechanisms of oxygen toxicity. Physiol Rev. 1968 Apr;48(2):311–373. doi: 10.1152/physrev.1968.48.2.311. [DOI] [PubMed] [Google Scholar]
- Hirai K. Comparison between 3,3'-diaminobenzidine and auto-oxidized 3,3 '-diaminobenzidine in the cytochemical demonstration of oxidative enzymes. J Histochem Cytochem. 1971 Jul;19(7):434–442. doi: 10.1177/19.7.434. [DOI] [PubMed] [Google Scholar]
- Karnovsky M. J., Rice D. F. Exogenous cytochrome c as an ultrastructural tracer. J Histochem Cytochem. 1969 Nov;17(11):751–753. doi: 10.1177/17.11.751. [DOI] [PubMed] [Google Scholar]
- Katzenstein A. L., Bloor C. M., Leibow A. A. Diffuse alveolar damage--the role of oxygen, shock, and related factors. A review. Am J Pathol. 1976 Oct;85(1):209–228. [PMC free article] [PubMed] [Google Scholar]
- Kistler G. S., Caldwell P. R., Weibel E. R. Development of fine structural damage to alveolar and capillary lining cells in oxygen-poisoned rat lungs. J Cell Biol. 1967 Mar;32(3):605–628. doi: 10.1083/jcb.32.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEMPAK J. G., WARD R. T. AN IMPROVED STAINING METHOD FOR ELECTRON MICROSCOPY. J Cell Biol. 1964 Sep;22:697–701. doi: 10.1083/jcb.22.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpelli E. M., Wolfson D. R., Colacicco G. Protein and lipid-protein fractions of lung washings: immunological characterization. J Appl Physiol. 1973 Jun;34(6):750–753. doi: 10.1152/jappl.1973.34.6.750. [DOI] [PubMed] [Google Scholar]
- Swartz H. M. Toxic oxygen effects. Int Rev Cytol. 1973;35:321–343. doi: 10.1016/s0074-7696(08)60358-7. [DOI] [PubMed] [Google Scholar]
- Taylor A. E., Gaar K. A., Jr Estimation of equivalent pore radii of pulmonary capillary and alveolar membranes. Am J Physiol. 1970 Apr;218(4):1133–1140. doi: 10.1152/ajplegacy.1970.218.4.1133. [DOI] [PubMed] [Google Scholar]