Abstract
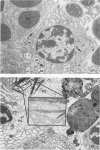
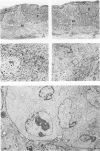
In this paper regional lymph nodes draining tumors and also nonregional lymph nodes have been studied at the light- and electron-microscopic levels. These nodes were obtained from rats bearing long-evolving autochthonous breast cancers. They were compared with a control group of the same age. A morphometric quantitative analysis was done to evaluate immunologically competent cell populations. In the experimental group there were no differences between regional and distal lymph nodes in the tumor challenge. However, when the experimental group was compared with the control group, differences appeared. The changes involved were diminution of the paracortical area with stimulation of plasma cells in the medullary cords. Ultrastructurally, in lymph nodes of the experimental group there were lymphoblasts in the paracortex and germinal centers, suggesting active lymphopoiesis. In addition, many plasma cells showed morphologic evidence of involution or other alterations. It is suggested that regional and distal lymph nodes have similar morphologic behavior when the tumor grows for a long period of time, a finding that contributes to our knowledge of the systemic implications of the lymphatic system.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Ito T. Qualitative and quantitative morphologic study of Peyer's patches of the mouse after neonatal thymectomy and hydrocortisone injection. Am J Anat. 1978 Feb;151(2):227–237. doi: 10.1002/aja.1001510206. [DOI] [PubMed] [Google Scholar]
- BLACK M. M., KERPE S., SPEER F. D. Lymph node structure in patients with cancer of the breast. Am J Pathol. 1953 May-Jun;29(3):505–521. [PMC free article] [PubMed] [Google Scholar]
- Badea E., Donovan G., Dumitrescu R., Fadel L., Popp I. Some data concerning immune processes in concomitant tumor immunity experimental models. Comparative in vivo and in vitro investigation I. In vivo experiments. Neoplasma. 1977;24(3):295–301. [PubMed] [Google Scholar]
- Black M. M., Leis H. P., Jr Cellular responses to autologous breast cancer tissue. Sequential observations. Cancer. 1973 Aug;32(2):384–389. doi: 10.1002/1097-0142(197308)32:2<384::aid-cncr2820320215>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Carr I., Underwood J. C., McGinty F., Wood P. The ultrastructure of the local lymphoreticular response to an experimental neoplasm. J Pathol. 1974 Jul;113(3):175–182. doi: 10.1002/path.1711130307. [DOI] [PubMed] [Google Scholar]
- Clifton K. H., Douple E. B., Sridharan B. N. Effects of grafts of single anterior pituitary glands on the incidence and type of mammary neoplasm in neutron- or gamma-irradiated Fischer female rats. Cancer Res. 1976 Oct;36(10):3732–3735. [PubMed] [Google Scholar]
- Cottier H., Turk J., Sobin L. A proposal for a standardized system of reporting human lymph node morphology in relation to immunological function. Bull World Health Organ. 1972;47(3):375–417. [PMC free article] [PubMed] [Google Scholar]
- Crile G., Jr The effect on metastasis of removing or irradiating regional nodes of mice. Surg Gynecol Obstet. 1968 Jun;126(6):1270–1272. [PubMed] [Google Scholar]
- Edwards A. J., Sumner M. R., Rowland G. F., Hurd C. M. Changes in lymphoreticular tissues during growth of a murine adenocarcinoma. I. Histology and weight of lymph nodes, spleen, and thymus. J Natl Cancer Inst. 1971 Aug;47(2):301–311. [PubMed] [Google Scholar]
- Eremin O., Plumb D., Coombs R. R. T and B lymphocyte populations in human normal lymph node, regional tumour lymph node and inflammatory lymph node. Int Arch Allergy Appl Immunol. 1976;52(1-4):277–290. doi: 10.1159/000231693. [DOI] [PubMed] [Google Scholar]
- Fisher B., Saffer E., Fisher E. R. Studies concerning the regional lymph node in cancer. IV. Tumor inhibition by regional lymph node cells. Cancer. 1974 Mar;33(3):631–636. doi: 10.1002/1097-0142(197403)33:3<631::aid-cncr2820330307>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Fisher E. R., Fisher B., Saffer E. The regional lymph node in cancer. Relationship of nodal histologic findings to cytotoxicity and immunity. Arch Pathol Lab Med. 1977 Mar;101(3):152–155. [PubMed] [Google Scholar]
- Fisher E. R., Reidbord H. E., Fisher B. Studies concerning the regional lymph node in cancer. V. Histologic and ultrastructural findings in regional and nonregional nodes. Lab Invest. 1973 Jan;28(1):126–133. [PubMed] [Google Scholar]
- Friedman D., Globerson A. Immune reactivity during aging. I. T-helper dependent and independent antibody responses to different antigens, in vivo and in vitro. Mech Ageing Dev. 1978 Apr;7(4):289–298. doi: 10.1016/0047-6374(78)90072-6. [DOI] [PubMed] [Google Scholar]
- Friedman D., Globerson A. Immune reactivity during aging. II. Analysis of the cellular mechanisms involved in the deficient antibody response in old mice. Mech Ageing Dev. 1978 Apr;7(4):299–307. doi: 10.1016/0047-6374(78)90073-8. [DOI] [PubMed] [Google Scholar]
- Goidl E. A., Innes J. B., Weksler M. E. Immunological studies of aging. II. Loss of IgG and high avidity plaque-forming cells and increased suppressor cell activity in aging mice. J Exp Med. 1976 Oct 1;144(4):1037–1048. doi: 10.1084/jem.144.4.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., McGregor D. D. Anatomical distribution of T and B lymphocytes in the rat. Development of lymphocyte-specific antisera. J Exp Med. 1973 Dec 1;138(6):1443–1465. doi: 10.1084/jem.138.6.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschl S., Black M. M., Kwon C. S. Ultrastructural characteristics of sinus histiocytic reaction in lymph nodes draining various stages of breast cancer. Cancer. 1976 Aug;38(2):807–817. doi: 10.1002/1097-0142(197608)38:2<807::aid-cncr2820380224>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Howard J. C., Hunt S. V., Gowans J. L. Identification of marrow-derived and thymus-derived small lymphocytes in the lymphoid tissue and thoracic duct lymph of normal rats. J Exp Med. 1972 Feb 1;135(2):200–219. doi: 10.1084/jem.135.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. A., Robinson G., Rees R. C., Baldwin R. W. Immune response of the draining and distal lymph nodes during the progressive grwoth of a chemically-induced transplantable rat hepatoma. Int J Cancer. 1978 Feb 15;21(2):171–178. doi: 10.1002/ijc.2910210208. [DOI] [PubMed] [Google Scholar]
- Kalderon A. E., Bogaars H. A., Diamond I., Cummings F. J., Kaplan S. R., Calabresi P. Ultrastructure of myeloma cells in a case with crystalcryoglobulinemia. Cancer. 1977 Apr;39(4):1475–1481. doi: 10.1002/1097-0142(197704)39:4<1475::aid-cncr2820390419>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Kikuchi K., Ishii Y., Ueno H., Koshiba H. Cell-mediated immunity involved in autochthonous tumor rejection in rats. Ann N Y Acad Sci. 1976;276:188–206. doi: 10.1111/j.1749-6632.1976.tb41646.x. [DOI] [PubMed] [Google Scholar]
- Meyer J. D., Meyer J. A. Prevention by splenectomy of thymus weight depletion in the presence of progressively growing tumor in rats: Brief communication. J Natl Cancer Inst. 1977 Sep;59(3):1023–1026. doi: 10.1093/jnci/59.3.1023. [DOI] [PubMed] [Google Scholar]
- Papaioannou A. N., Tsakraklides V., Critselis A. N., Good R. A. The thymus in breast cancer: observations in 25 patients and in controls. Cancer. 1978 Mar;41(3):790–796. doi: 10.1002/1097-0142(197803)41:3<790::aid-cncr2820410302>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Parrott D. V., De Sousa M. A., East J. Thymus-dependent areas in the lymphoid organs of neonatally thymectomized mice. J Exp Med. 1966 Jan 1;123(1):191–204. doi: 10.1084/jem.123.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitot H. C. Carcinogenesis and aging--two related phenomena? A review. Am J Pathol. 1977 May;87(2):444–472. [PMC free article] [PubMed] [Google Scholar]
- Satodate R., Sasou S., Katsura S., Yoshida M., Hirata M. Vergleichende histologische-morphometrische Untersuchung der Marginalzone und des Keimzentrums der Milzfollikel der Ratte nach Endotoxininjektion. Exp Pathol (Jena) 1977 Jul-Aug;14(1-2):100–107. [PubMed] [Google Scholar]
- Sprent J. Circulating T and B lymphocytes of the mouse. I. Migratory properties. Cell Immunol. 1973 Apr;7(1):10–39. doi: 10.1016/0008-8749(73)90180-9. [DOI] [PubMed] [Google Scholar]
- Tsakraklides V., Tsakraklides E., Good R. A. An autopsy study of human axillary lymph node histology. Am J Pathol. 1975 Jan;78(1):7–22. [PMC free article] [PubMed] [Google Scholar]
- WELSH R. A. Electron microscopic localization of Russell bodies in the human plasma cell. Blood. 1960 Sep;16:1307–1312. [PubMed] [Google Scholar]
- Wanebo H. J., Thaler H. T., Hansen J. A., Rosen P. P., Robbins G. F., Urban J. A., Oettgen H. F., Good R. A. Immunologic reactivity in patients with primary operable breast cancer. Cancer. 1978 Jan;41(1):84–94. doi: 10.1002/1097-0142(197801)41:1<84::aid-cncr2820410113>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Wang D. Y., Goodwin P. R., Bulbrook R. D., Hayward J. L. Plasma immunoglobulin levels in patients with breast cancer. Cancer. 1977 May;39(5):2190–2193. doi: 10.1002/1097-0142(197705)39:5<2190::aid-cncr2820390536>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Weissman I. L., Warnke R., Butcher E. C., Rouse R., Levy R. The lymphoid system. Its normal architecture and the potential for understanding the system through the study of lymphoproliferative diseases. Hum Pathol. 1978 Jan;9(1):25–45. doi: 10.1016/s0046-8177(78)80005-7. [DOI] [PubMed] [Google Scholar]
- Welsch C. W., Jenkins T. W., Meites J. Increased incidence of mammary tumors in the female rat grafted with multiple pituitaries. Cancer Res. 1970 Apr;30(4):1024–1029. [PubMed] [Google Scholar]
- Zöller M., Price M. R., Baldwin R. W. Cell-mediated cytotoxicity to chemically-induced rat tumours. Int J Cancer. 1975 Oct 15;16(4):593–606. doi: 10.1002/ijc.2910160409. [DOI] [PubMed] [Google Scholar]
- Zöller M., Price M. R., Baldwin R. W. Inhibition of cell-mediated cytotoxicity to chemically induced rat tumours by soluble tumour and embryo cell extracts. Int J Cancer. 1976 Jan 15;17(1):129–137. doi: 10.1002/ijc.2910170117. [DOI] [PubMed] [Google Scholar]
- van de Velde C. J., Meyer C. J., Cornellsse C. J., van der Velde E. A., van Putten L. M., Swaveling A. A morphometrical analysis of lymph node responses to tumors of different immunogenicity. Cancer Res. 1978 Mar;38(3):661–667. [PubMed] [Google Scholar]