Abstract
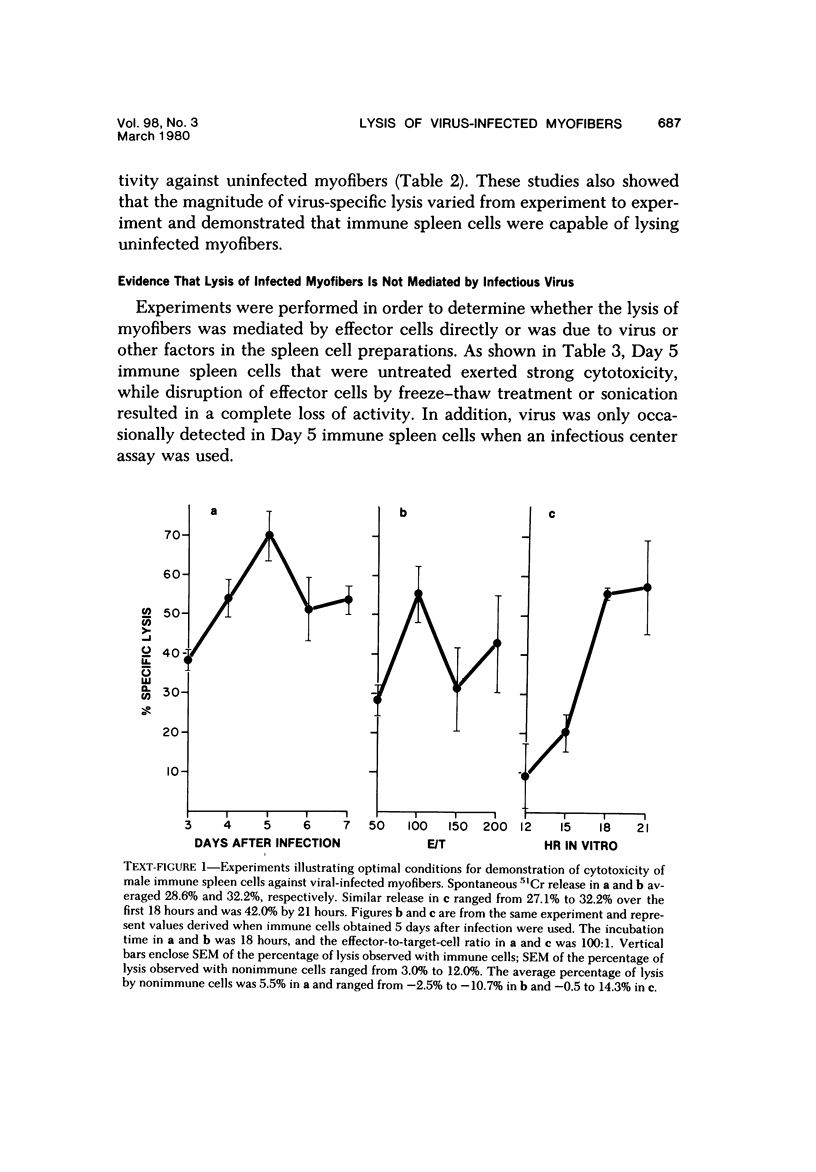
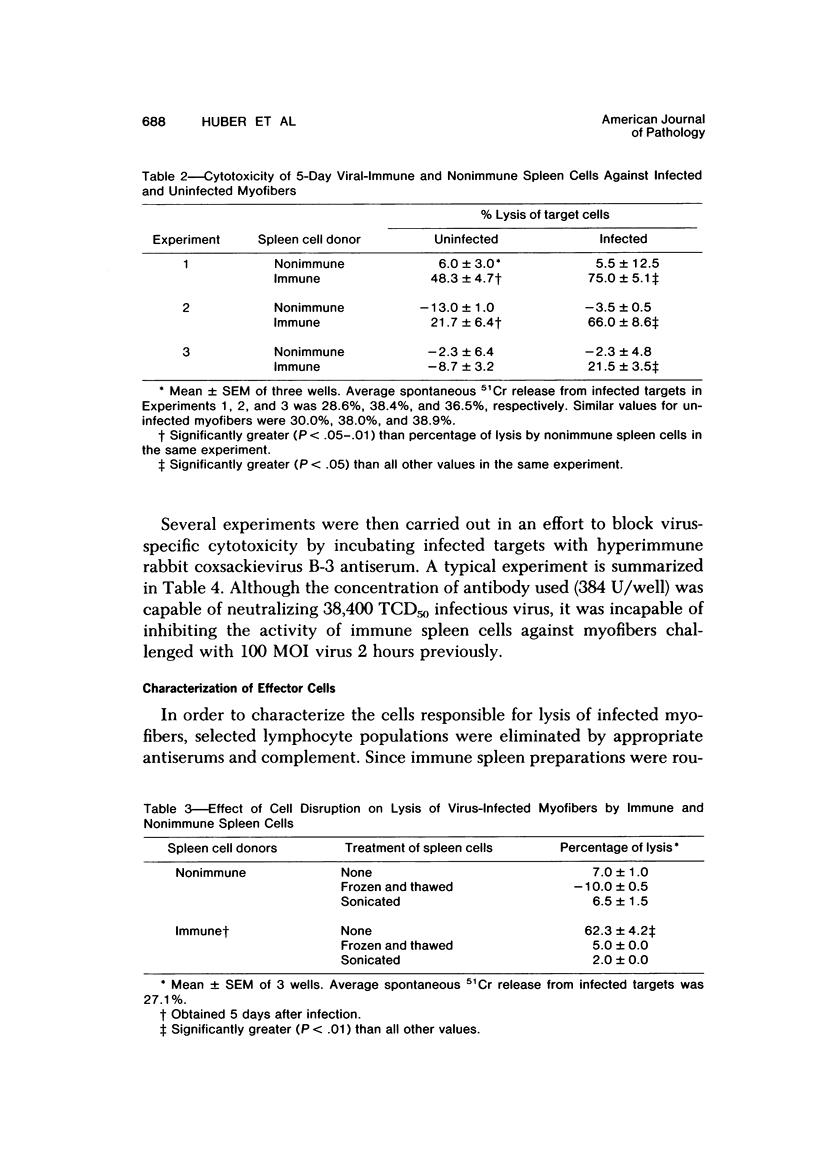
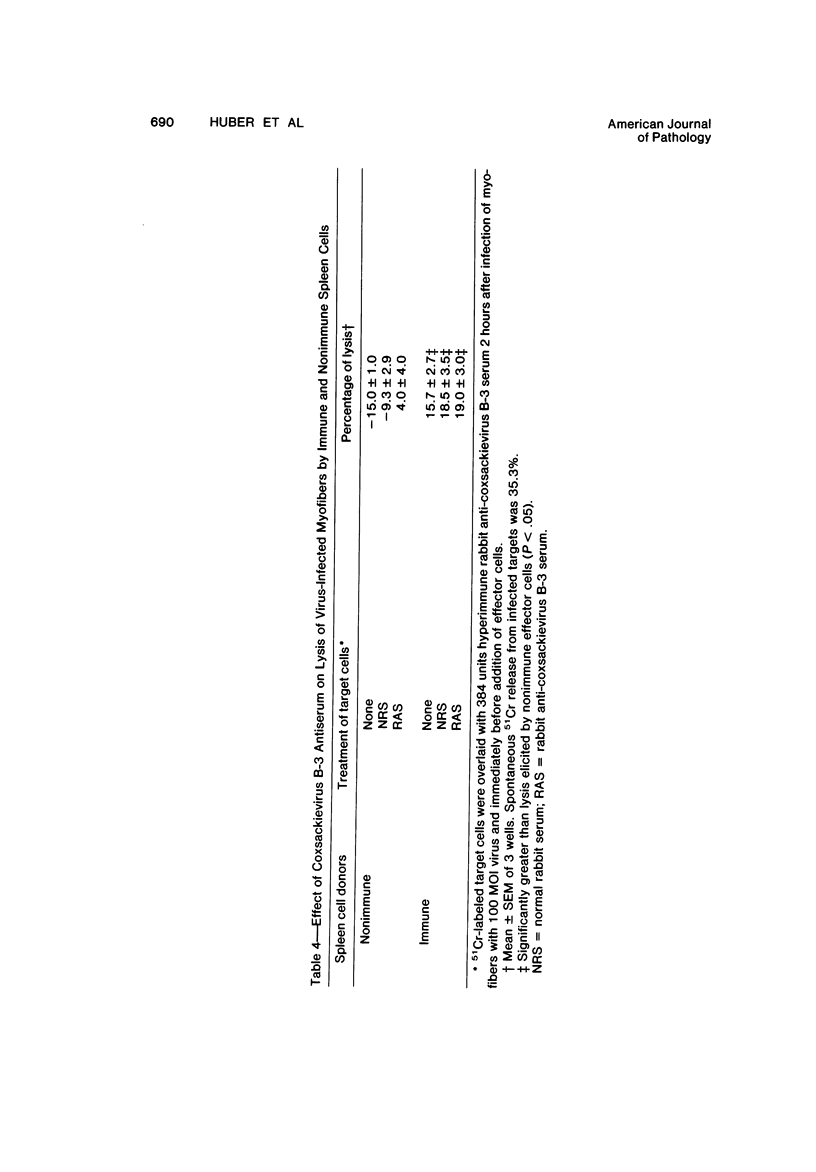
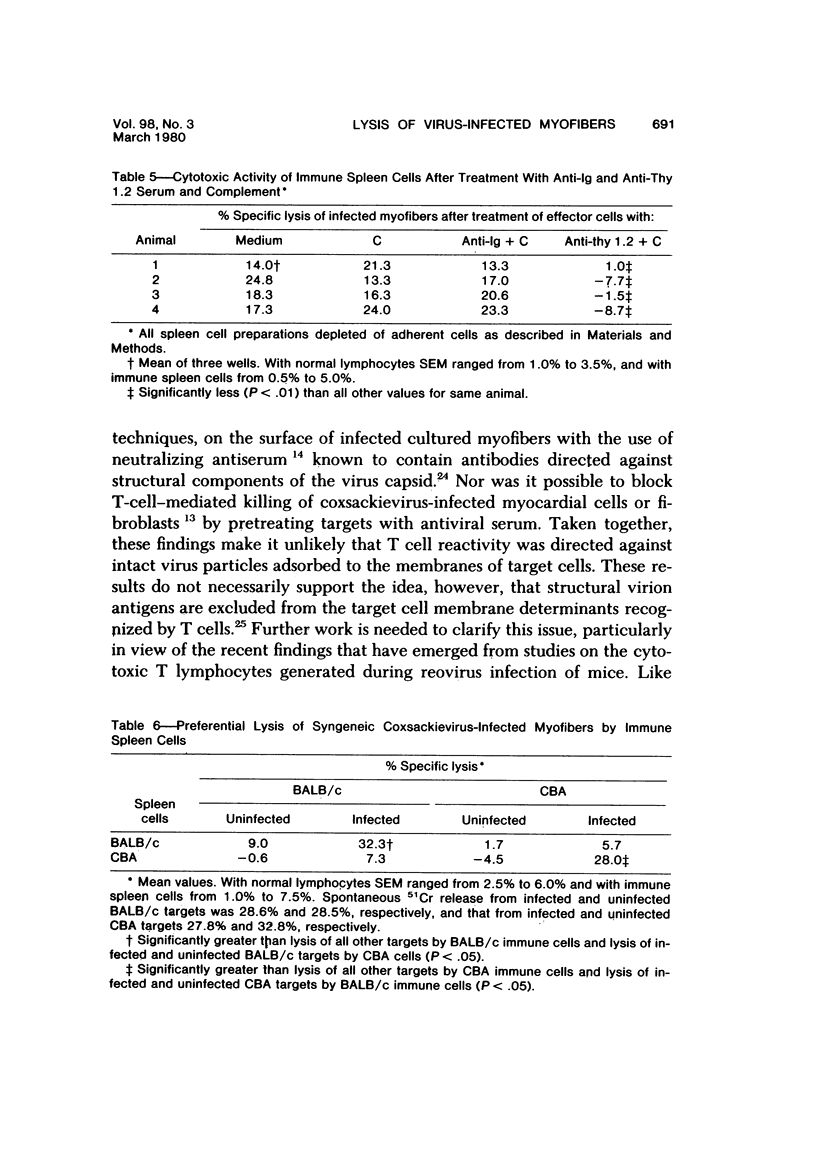
Spleen cells from male adult BALB/c mice given intraperitoneal injections of purified coxsackievirus B-3 were examined for the ability to lyse syngeneic neonatal myofibers in culture. Cytotoxicity against infected and uninfected targets was measured with the use of an in vitro 51Cr release assay. Immune spleen cells obtained 4--7 days after infection were cytotoxic for viral-infected myofibers. Peak reactivity was observed 5 days after infection. At this time immune spleen cells showed significantly less reactivity against uninfected myofibers. Cytotoxicity against infected targets was mediated by T lymphocytes, since reactivity was abolished by treatment with anti-thy 1.2 and complement. Treatment with anti-Ig and complement caused no loss of activity. Reciprocal assays performed with BALB/c and CBA cells showed that maximal cytotoxicity occurred against infected syngeneic myofibers, providing further evidence that viral-specific effector cells were T lymphocytes. In addition, hyperimmune rabbit anti-coxsackievirus B-3 antiserum could not block immune spleen cell lysis of infected targets, suggesting that coxsackievirus-infected myofibers expressed surface membrane antigens not recognized by specific neutralizing antibody.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CROWELL R. L., SYVERTON J. T. The mammalian cell-virus relationship. VI. Sustained infection of HeLa cells by Coxsackie B3 virus and effect on superinfection. J Exp Med. 1961 Feb 1;113:419–435. doi: 10.1084/jem.113.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Blanden R. V., Zinkernagel R. M. Specificity of virus-immune effector T cells for H-2K or H-2D compatible interactions: implications for H-antigen diversity. Transplant Rev. 1976;29:89–124. doi: 10.1111/j.1600-065x.1976.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Evans R., Alexander P. Mechanism of immunologically specific killing of tumour cells by macrophages. Nature. 1972 Mar 24;236(5343):168–170. doi: 10.1038/236168a0. [DOI] [PubMed] [Google Scholar]
- Finberg R., Weiner H. L., Fields B. N., Benacerraf B., Burakoff S. J. Generation of cytolytic T lymphocytes after reovirus infection: role of S1 gene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):442–446. doi: 10.1073/pnas.76.1.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRODUMS E. I., DEMPSTER G. Myocarditis in experimental Coxsackie B-3 infection. Can J Microbiol. 1959 Dec;5:605–615. doi: 10.1139/m59-074. [DOI] [PubMed] [Google Scholar]
- Godman G. C. The cytopathology of enteroviral infection. Int Rev Exp Pathol. 1966;5:67–110. [PubMed] [Google Scholar]
- Kiessling R., Klein E., Pross H., Wigzell H. "Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975 Feb;5(2):117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- Lerner A. M., Wilson F. M. Virus myocardiopathy. Prog Med Virol. 1973;15:63–91. [PubMed] [Google Scholar]
- Oberg B., Philipson L. Replication of poliovirus RNA studied by gel filtration and electrophoresis. Eur J Biochem. 1969 Dec;11(2):305–315. doi: 10.1111/j.1432-1033.1969.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Virus neutralization and virus-induced immune complex disease. Virus-antibody union resulting in immunoprotection or immunologic injury--two sides of the same coin. Prog Med Virol. 1975;19:84–119. [PubMed] [Google Scholar]
- Paque R. E., Gauntt C. J., Nealon T. J., Trousdale M. D. Assessment of cell-mediated hypersensitivity against coxsackievirus B3 viral-induced myocarditis utilizing hypertonic salt extracts of cardiac tissue. J Immunol. 1978 May;120(5):1672–1678. [PubMed] [Google Scholar]
- Perlmann P., Holm G. Cytotoxic effects of lymphoid cells in vitro. Adv Immunol. 1969;11:117–193. doi: 10.1016/s0065-2776(08)60479-4. [DOI] [PubMed] [Google Scholar]
- Roesing T. G., Landau B. J., Crowell R. L. Limited persistence of viral antigen in coxsackievirus B3 induced heart disease in mice. Proc Soc Exp Biol Med. 1979 Mar;160(3):382–386. doi: 10.3181/00379727-160-40455. [DOI] [PubMed] [Google Scholar]
- Sainani G. S., Krompotic E., Slodki S. J. Adult heart disease due to the Coxsackie virus B infection. Medicine (Baltimore) 1968 Mar;47(2):133–147. doi: 10.1097/00005792-196803000-00003. [DOI] [PubMed] [Google Scholar]
- Smith W. G. Coxsackie B myopericarditis in adults. Am Heart J. 1970 Jul;80(1):34–46. doi: 10.1016/0002-8703(70)90035-9. [DOI] [PubMed] [Google Scholar]
- Wong C. Y., Woodruff J. J., Woodruff J. F. Generation of cytotoxic T lymphocytes during coxsackievirus B-3 infection. I. Model and viral specificity1. J Immunol. 1977 Apr;118(4):1159–1164. [PubMed] [Google Scholar]
- Wong C. Y., Woodruff J. J., Woodruff J. F. Generation of cytotoxic T lymphocytes during coxsackievirus B-3 infection. III. Role of sex. J Immunol. 1977 Aug;119(2):591–597. [PubMed] [Google Scholar]
- Wong C. Y., Woodruff J. J., Woodruff J. F. Generation of cytotoxic T lymphocytes during coxsackievirus tb-3 infection. II. Characterization of effector cells and demonstration cytotoxicity against viral-infected myofibers1. J Immunol. 1977 Apr;118(4):1165–1169. [PubMed] [Google Scholar]
- Woodruff J. F., Kilbourne E. D. The influence of quantitated post-weaning undernutrition on coxsackievirus B3 infection of adult mice. I. Viral persistence and increased severity of lesions. J Infect Dis. 1970 Feb;121(2):137–163. doi: 10.1093/infdis/121.2.137. [DOI] [PubMed] [Google Scholar]
- Woodruff J. F. The influence of quantitated post-weaning undernutrition on coxsackievirus B3 infection of adult mice. II. Alteration of host defense mechanisms. J Infect Dis. 1970 Feb;121(2):164–181. doi: 10.1093/infdis/121.2.164. [DOI] [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. Involvement of T lymphocytes in the pathogenesis of coxsackie virus B3 heart disease. J Immunol. 1974 Dec;113(6):1726–1734. [PubMed] [Google Scholar]



