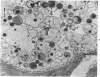
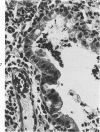
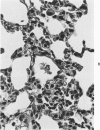
Abstract
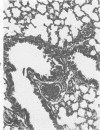
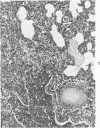
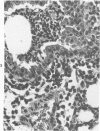
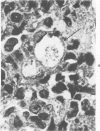
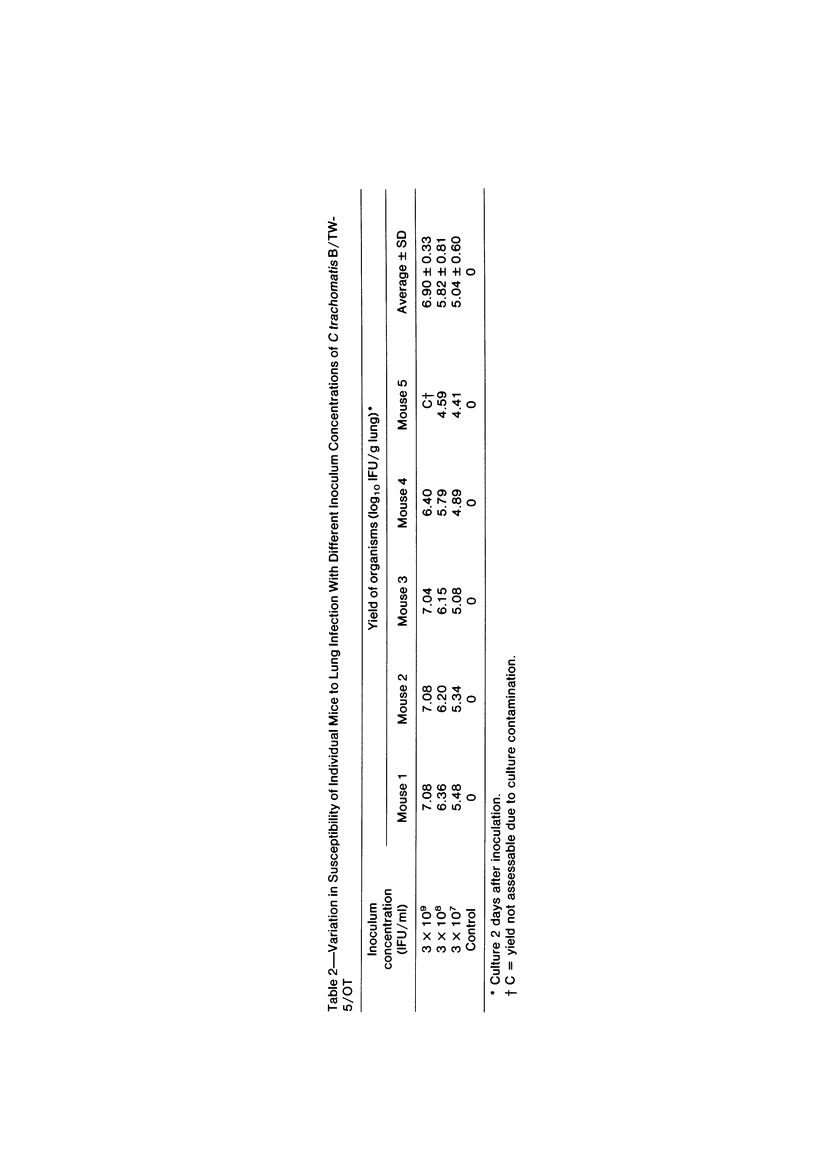
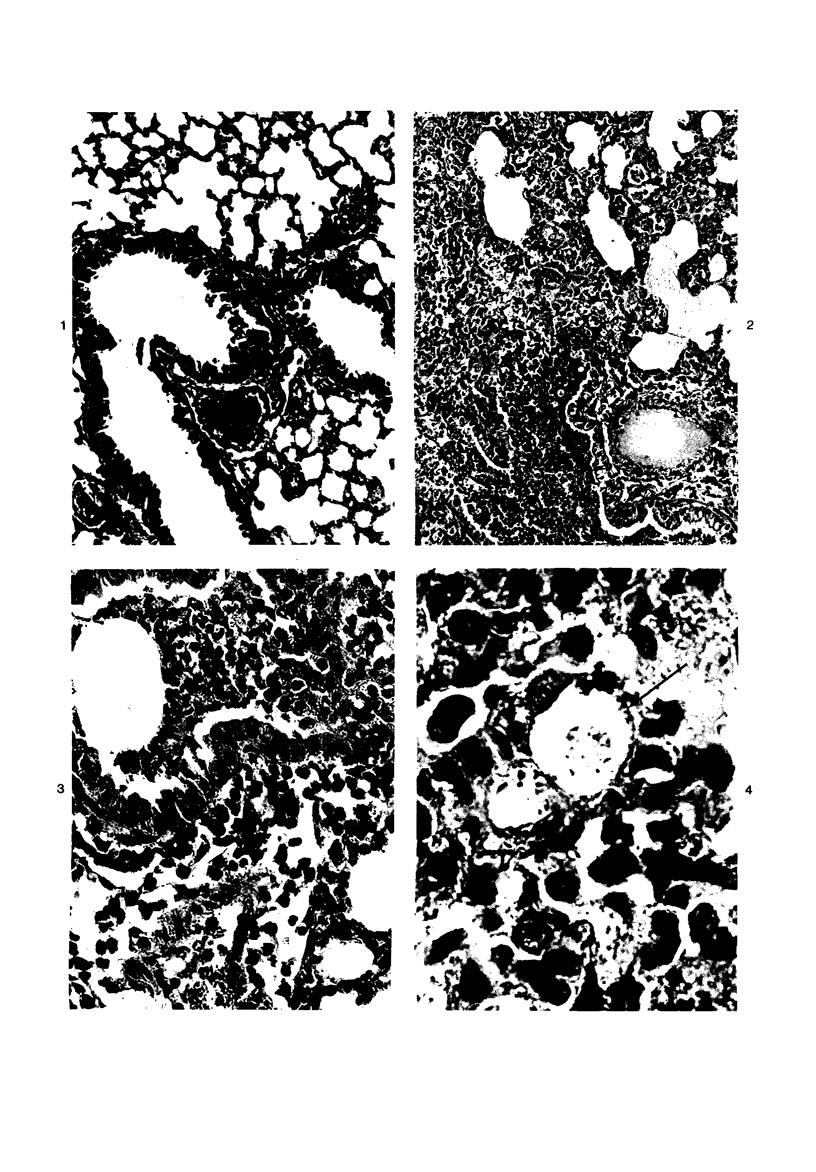
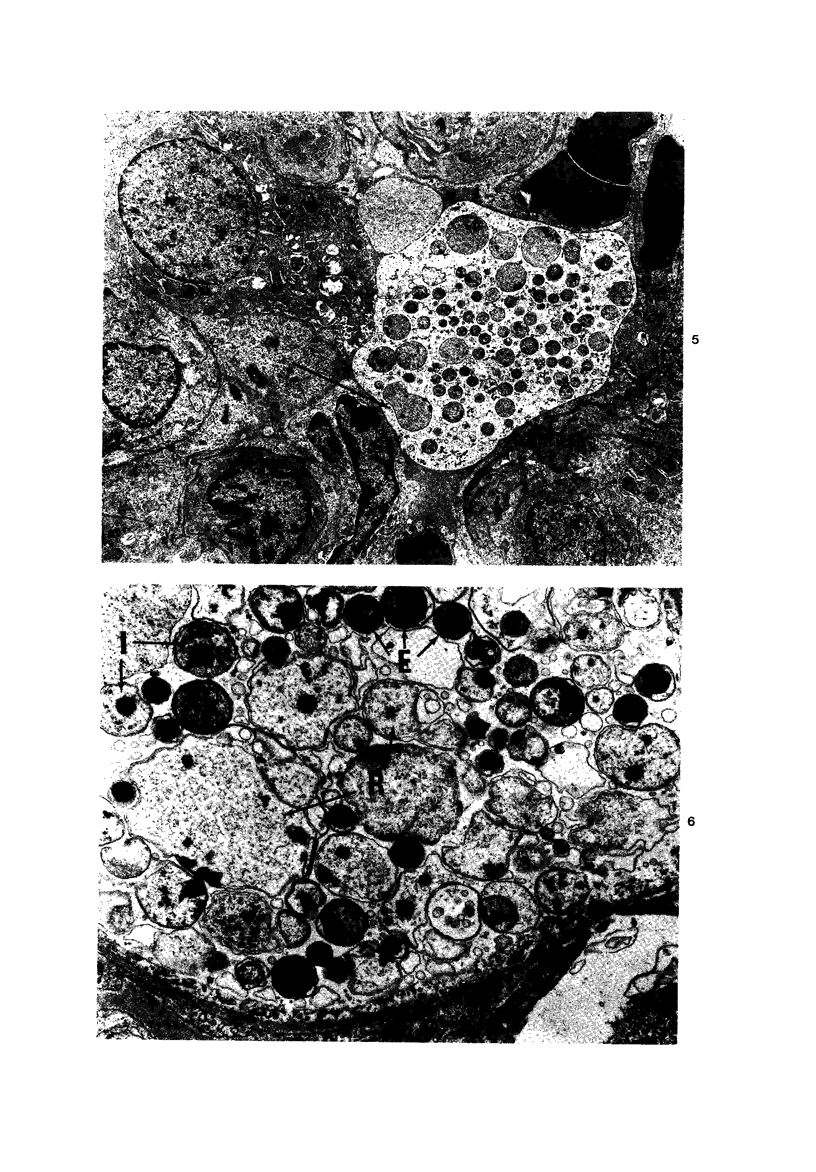
Swiss-Webster white mice were infected with Chlamydia trachomatis organisms through intranasal inoculation. It was found that a typical interstitial pneumonitis could be induced. Histopathologic findings showed that the lung infiltration was predominantly polymorphonuclear cells and was most prominant on Day 2. The cellular infiltrate gradually changed to mononuclear cells after Day 3. Intracytoplasmic inclusions were frequently found in the interstitial cells and occasionally in the bronchial epithelial cells. Typical chlamydial bodies (elementary, intermediate, and reticulate forms) were identified by electron microscopy. The organisms were recovered from mouse lungs on Days 1--7, with the highest yields on Day 2. This correlated with the peak of lung infiltration seen by histologic examination. Antibodies specific to the infecting immunotype began to appear between Day 7 and Day 10 after inoculation and lasted until Day 35 without a decline in titers. A delayed hypersensitivity reaction was observed by footpad test from Day 5 to Day 21, with the peak reaction at Day 7. This study showed that the mouse model could be used to study the immunopathogenesis of C trachomatis infection.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander E. R., Wang S. P., Grayston J. T. Further classification of TRIC agents from ocular trachoma and other sources by the mouse toxicity prevention test. Am J Ophthalmol. 1967 May;63(5 Suppl):1469–1478. doi: 10.1016/0002-9394(67)94133-5. [DOI] [PubMed] [Google Scholar]
- Arth C., Von Schmidt B., Grossman M., Schachter J. Chlamydial pneumonitis. J Pediatr. 1978 Sep;93(3):447–449. doi: 10.1016/s0022-3476(78)81155-x. [DOI] [PubMed] [Google Scholar]
- BERNKOPF H. The susceptibility of white mice to a strain of trachoma virus and their use in neutralization tests. Bull Res Counc Isr Sect E Exp Med. 1959 Oct;8E:25–29. [PubMed] [Google Scholar]
- Beem M. O., Saxon E. M. Respiratory-tract colonization and a distinctive pneumonia syndrome in infants infected with Chlamydia trachomatis. N Engl J Med. 1977 Feb 10;296(6):306–310. doi: 10.1056/NEJM197702102960604. [DOI] [PubMed] [Google Scholar]
- Bell S. D., Fraser C. E. Experimental trachoma in owl monkeys. Am J Trop Med Hyg. 1969 Jul;18(4):568–572. doi: 10.4269/ajtmh.1969.18.568. [DOI] [PubMed] [Google Scholar]
- GRAY D. F., JENNINGS P. A. Allergy in experimental mouse tuberculosis. Am Rev Tuberc. 1955 Aug;72(2):171–195. doi: 10.1164/artpd.1955.72.2.171. [DOI] [PubMed] [Google Scholar]
- Graham D. M. Growth and immunogenicity of TRIC agents in mice. Am J Ophthalmol. 1967 May;63(5 Suppl):1173–1190. doi: 10.1016/0002-9394(67)94100-1. [DOI] [PubMed] [Google Scholar]
- Graham D. M. Growth and neutralization of the trachoma agent in mouse lungs. Nature. 1965 Sep 25;207(5004):1379–1380. doi: 10.1038/2071379a0. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975 Jul;132(1):87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- HURST E. W., REEVE P. Transmission of the viruses of trachoma, inclusion blennorrhoea and lymphogranuloma to mice. Nature. 1960 Apr 23;186:336–336. doi: 10.1038/186336a0. [DOI] [PubMed] [Google Scholar]
- Harrison H. R., Alexander E. R., Chiang W. T., Giddens W. E., Jr, Boyce J. T., Benjamin D., Gale J. L. Experimental nasopharyngitis and pneumonia caused by Chlamydia trachomatis in infant baboons: histopathologic comparison with a case in a human infant. J Infect Dis. 1979 Feb;139(2):141–146. doi: 10.1093/infdis/139.2.141. [DOI] [PubMed] [Google Scholar]
- Harrison H. R., English M. G., Lee C. K., Alexander E. R. Chlamydia trachomatis infant pneumonitis: comparison with matched controls and other infant pneumonitis. N Engl J Med. 1978 Mar 30;298(13):702–708. doi: 10.1056/NEJM197803302981303. [DOI] [PubMed] [Google Scholar]
- Kuo C. C. Cultures of Chlamydia trachomatis in mouse peritoneal macrophages: factors affecting organism growth. Infect Immun. 1978 May;20(2):439–445. doi: 10.1128/iai.20.2.439-445.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Grayston T. Interaction of Chlamydia trachomatis organisms and HeLa 229 cells. Infect Immun. 1976 Apr;13(4):1103–1109. doi: 10.1128/iai.13.4.1103-1109.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C., Chen W. J. A mouse model of Chlamydia trachomatis pneumonitis. J Infect Dis. 1980 Feb;141(2):198–202. doi: 10.1093/infdis/141.2.198. [DOI] [PubMed] [Google Scholar]
- MITSUI Y., KAJIMA M., NISHIMURA A., KONISHI K. Morphology and developmental cycle of the trachoma agent. Morphology of trachoma agent in conjunctiva and chick embryo. Ann N Y Acad Sci. 1962 Mar 5;98:131–144. doi: 10.1111/j.1749-6632.1962.tb30538.x. [DOI] [PubMed] [Google Scholar]
- Ripa K. T., Møller B. R., Mårdh P. A., Freundt E. A., Melsen F. Experimental acute salpingitis in grivet monkeys provoked by Chlamydia trachomatis. Acta Pathol Microbiol Scand B. 1979 Feb;87B(1):65–70. doi: 10.1111/j.1699-0463.1979.tb02404.x. [DOI] [PubMed] [Google Scholar]
- Schachter J. Chlamydial infections (second of three parts). N Engl J Med. 1978 Mar 2;298(9):490–495. doi: 10.1056/NEJM197803022980905. [DOI] [PubMed] [Google Scholar]
- Schachter J., Meyer K. F. Lymphogranuloma venereum. II. Characterization of some recently isolated strains. J Bacteriol. 1969 Sep;99(3):636–638. doi: 10.1128/jb.99.3.636-638.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Eschenbach D. A., Alexander E. R., Holmes K. K. Light and electron microscopic study of Chlamydia trachomatis infection of the uterine cervix. J Infect Dis. 1975 Jun;131(6):678–687. doi: 10.1093/infdis/131.6.678. [DOI] [PubMed] [Google Scholar]
- WANG S., GRAYSTON J. T. Trachoma in the Taiwan monkey Macaca cyclopis. Ann N Y Acad Sci. 1962 Mar 5;98:177–187. doi: 10.1111/j.1749-6632.1962.tb30542.x. [DOI] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T., Gale J. L. Three new immunologic types of trachoma-inclusion conjunctivitis organisms. J Immunol. 1973 Mar;110(3):873–879. [PubMed] [Google Scholar]
- Wang S. P., Kuo C. C., Grayston J. T. A simplified method for immunological typing of trachoma-inclusion conjunctivitis-lymphogranuloma venereum organisms. Infect Immun. 1973 Mar;7(3):356–360. doi: 10.1128/iai.7.3.356-360.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]