Abstract
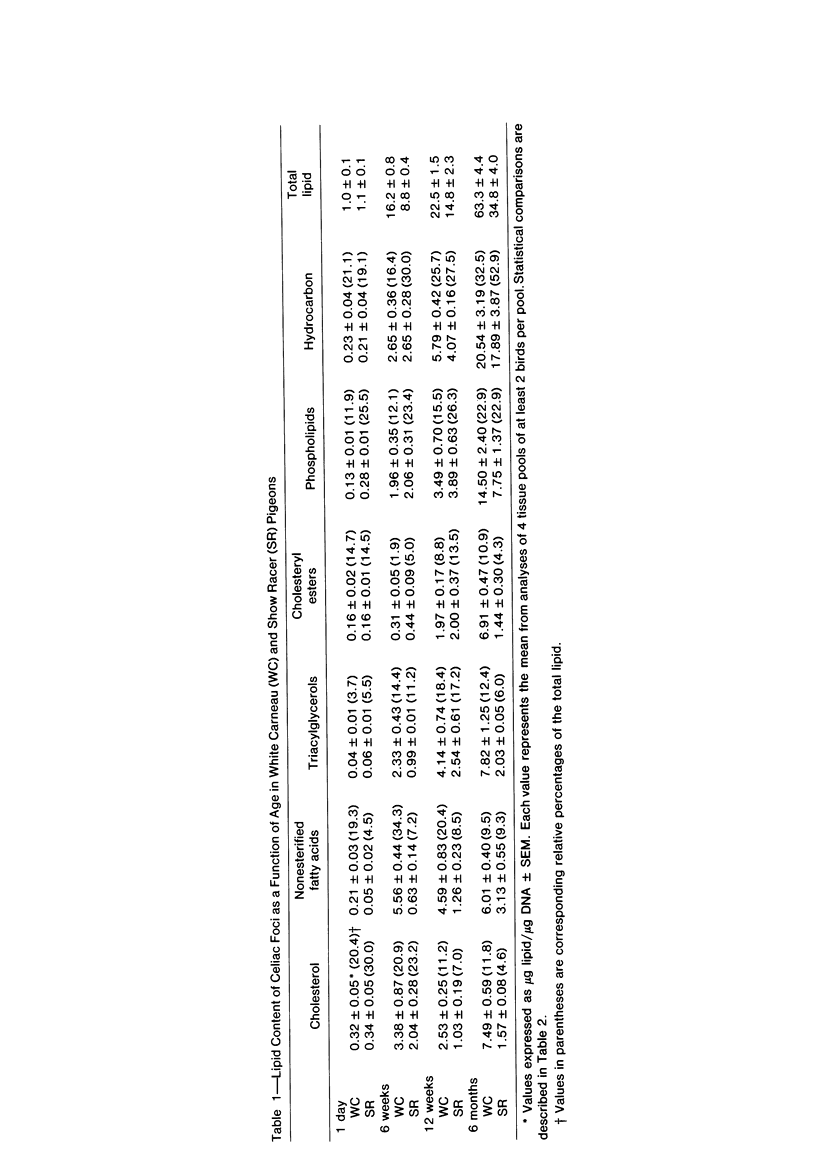
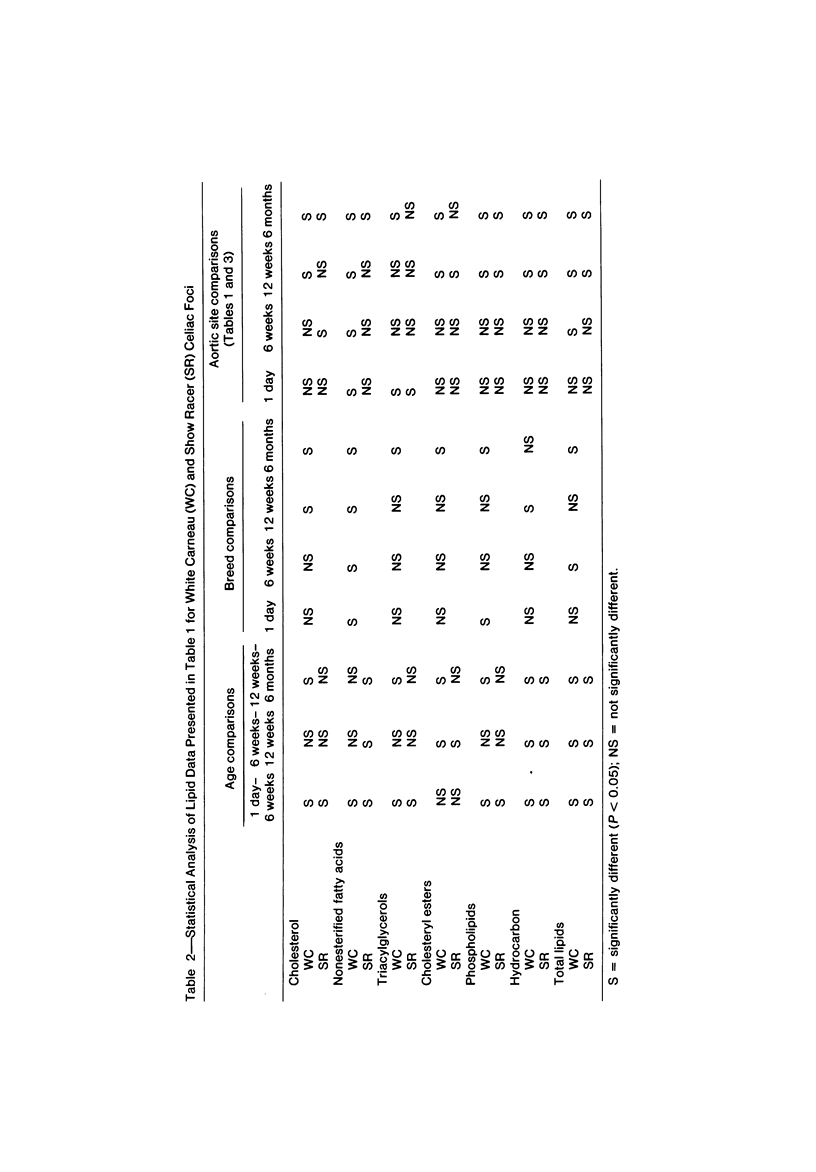
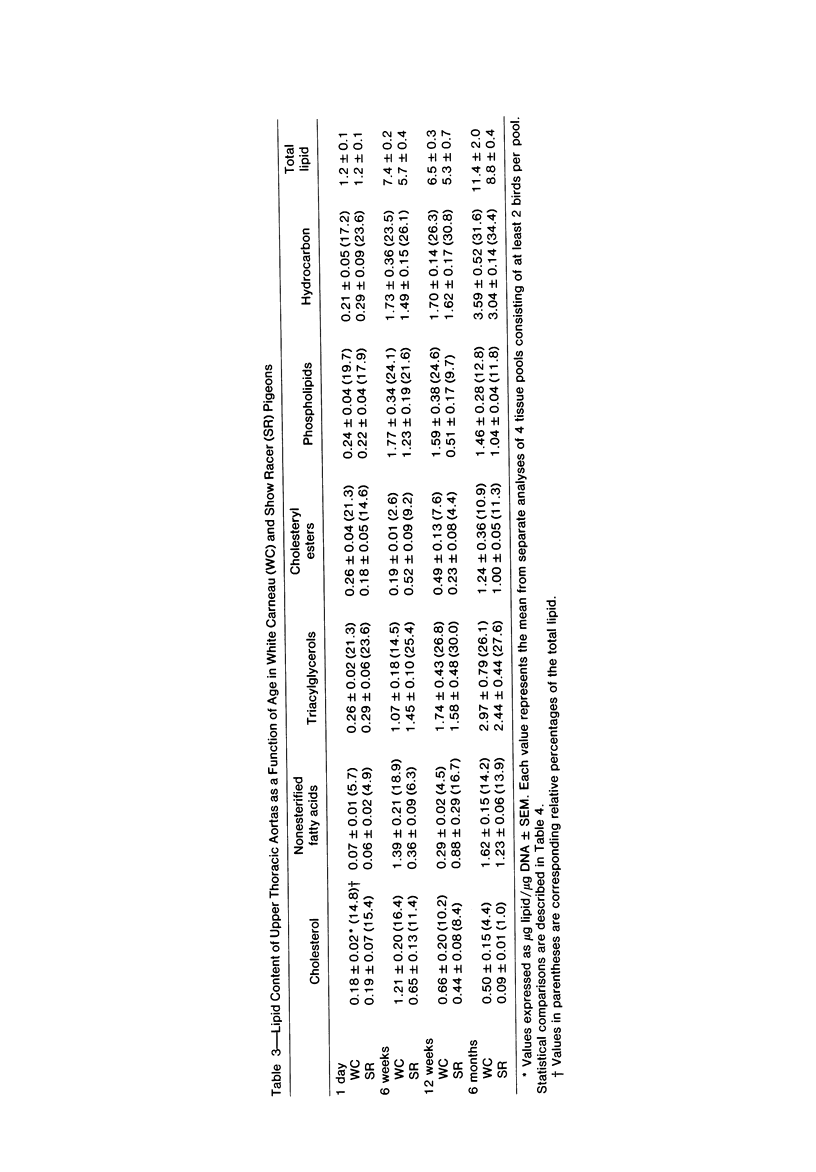
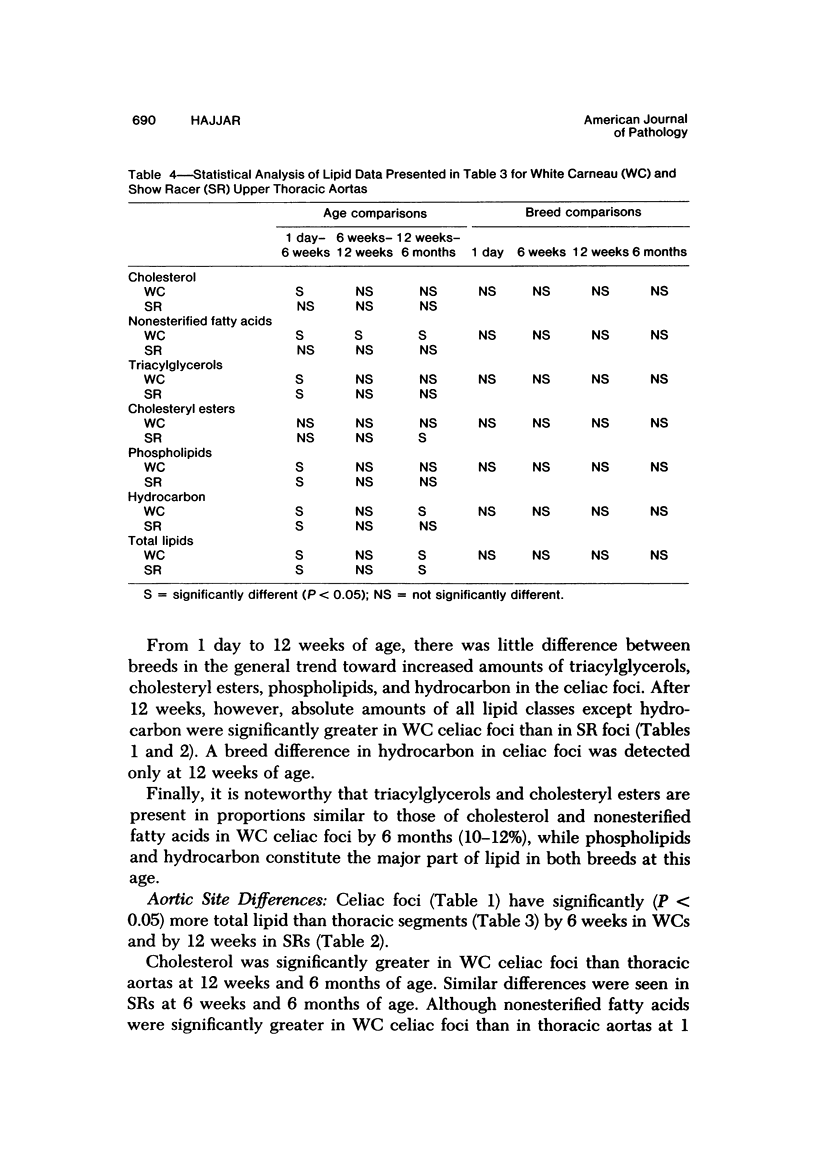
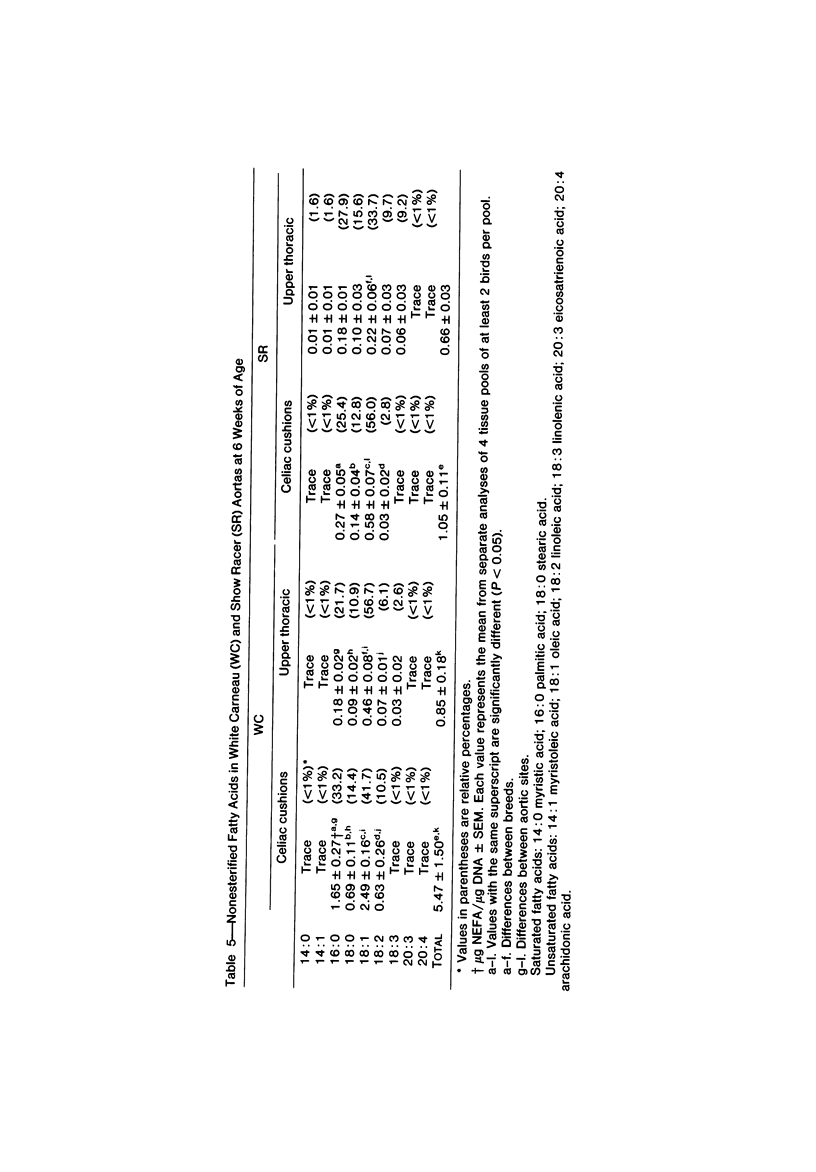
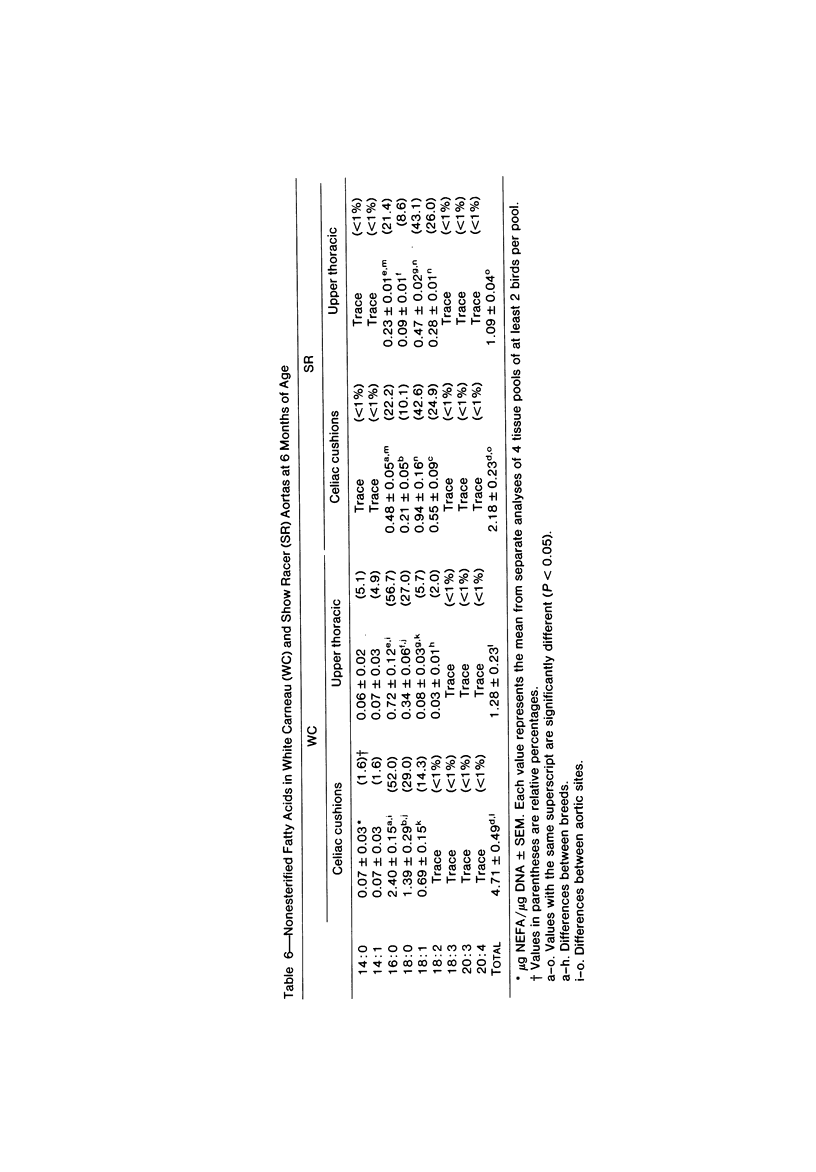
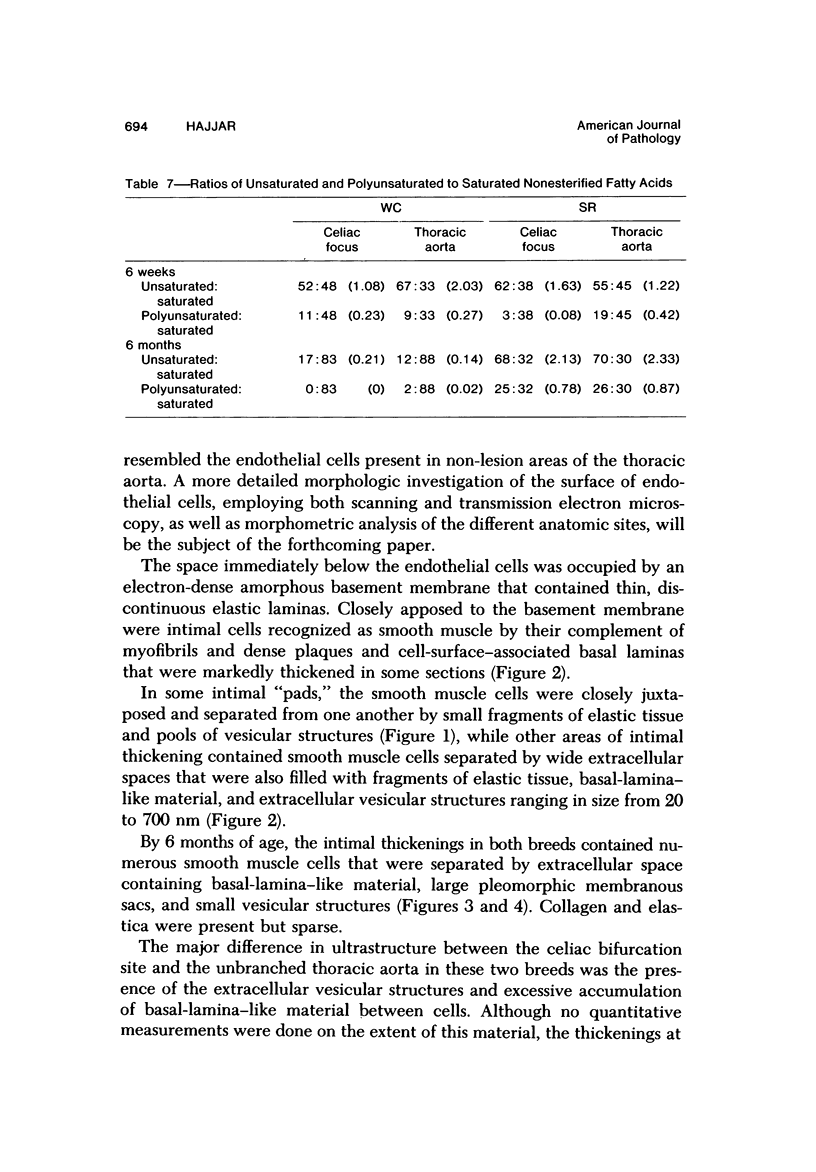
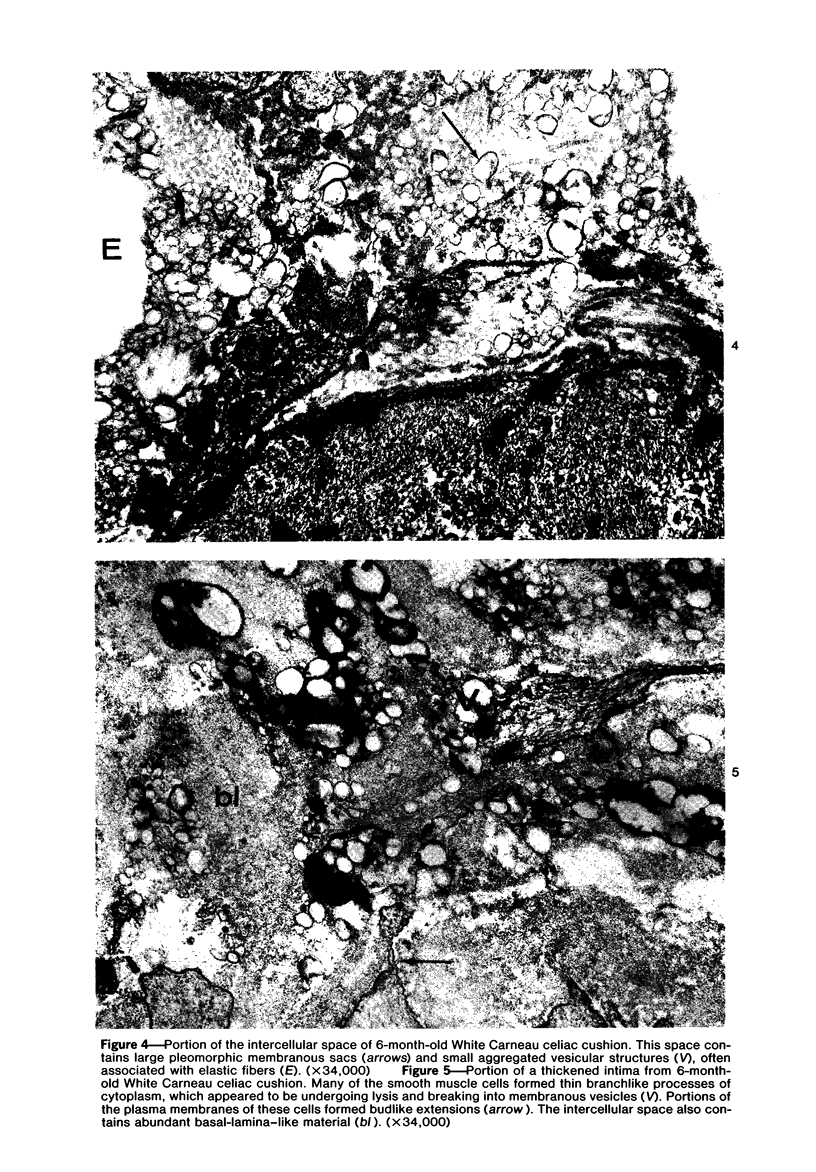
To identify the initial period and type of lipid accumulation during spontaneous atherosclerosis, quantitative chromatographic profiles of major lipid classes in upper thoracic aortas (non-lesion areas) and celiac artery cushions (lesion areas) were obtained from atherosclerosis-susceptible White Carneau (WC) and atherosclerosis-resistant Show Racer (SR) pigeons from 1 day to 6 months of age. Thoracic aortas of WC and SR pigeons contained similar amounts of cholesterol, nonesterified fatty acids, triacylglycerols, cholesteryl esters, phospholipids, and hydrocarbon at each age studied. However, celiac sites in WCs contained more total lipid than corresponding SR sites at 6 weeks and 6 months of age. This initial increase at 6 weeks in WCs was characterized by invreased concentrations of nonesterified saturated fatty acids. By 6 months of age, WC celiac cushions had greater concentrations of each lipid class except hydrocarbon than did SR cushions. This initial lipid accumulation was accompanied by ultrastructural changes within the arterial wall, which included the presence of extracellular, vesiclelike structures and extensive accumulation of basal lamina-like material between cells. This material was not present in aortic regions that are not predisposed to lesion formation. This material increased by 6 months of age in the enlarging WC fibromuscular intimal cushions. These morphologic changes paralleled the quantitative lipid increases and represented the first morphologic changes detectable at this site. Age-related changes in arterial lipid content and ultrastructure in SRs are different from those related to early spontaneous atherogenesis in WCs.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M., Koletsky S. Arteriosclerosis of the mesenteric arteries of rats with renal hypertension. Electron microscopic observations. Am J Pathol. 1970 Dec;61(3):293–322. [PMC free article] [PubMed] [Google Scholar]
- Berberian P. A., Ziboh V. A., Hsia S. L. Prostaglandin E2 biosynthesis: changes in rabbit aorta and skin during experimental atherogenesis. J Lipid Res. 1976 Jan;17(1):46–52. [PubMed] [Google Scholar]
- Bonner M. J., Miller B. F., Kothari H. V., Kritchevsky D. Lysosomal enzymes in aortas of species susceptible and resistant to atherosclerosis. Proc Soc Exp Biol Med. 1972 Apr;139(4):1359–1362. doi: 10.3181/00379727-139-36363. [DOI] [PubMed] [Google Scholar]
- CLARKSON T. B., PRICHARD R. W., NETSKY M. G., LOFLAND H. B. Atherosclerosis in pigeons; its spontaneous occurrence and resemblance to human atherosclerosis. AMA Arch Pathol. 1959 Aug;68(2):143–147. [PubMed] [Google Scholar]
- Chalvardjian A., Rudnicki E. Determination of lipid phosphorus in the nanomolar range. Anal Biochem. 1970 Jul;36(1):225–226. doi: 10.1016/0003-2697(70)90352-0. [DOI] [PubMed] [Google Scholar]
- Chan S. H., Higgins E., Jr Uncoupling activity of endogenous free fatty acids in rat liver mitochondria. Can J Biochem. 1978 Feb;56(2):111–116. doi: 10.1139/o78-018. [DOI] [PubMed] [Google Scholar]
- Clarkson T. B., King J. S., Jr, Lofland H. B., Feldner M. A., Bullock B. C. Pathologic characteristics and composition of diet-aggravated atherosclerotic plaques during "regression". Exp Mol Pathol. 1973 Dec;19(3):267–283. doi: 10.1016/0014-4800(73)90059-2. [DOI] [PubMed] [Google Scholar]
- Clarkson T. B., Prichard R. W., Bullock B. C., St Clair R. W., Lehner N. D., Jones D. C., Wagner W. D., Rudel L. L. Pathogenesis of artherosclerosis; some advances from using animal models. Exp Mol Pathol. 1976 Jun;24(3):264–286. doi: 10.1016/0014-4800(76)90065-4. [DOI] [PubMed] [Google Scholar]
- Cliff W. J. The aortic tunica media in aging rats. Exp Mol Pathol. 1970 Oct;13(2):172–189. doi: 10.1016/0014-4800(70)90004-3. [DOI] [PubMed] [Google Scholar]
- Cooke P. H., Smith S. C. Smooth muscle cells: the source of foam cells in atherosclerotic white Carneau pigeons. Exp Mol Pathol. 1968 Apr;8(2):171–189. doi: 10.1016/0014-4800(68)90014-2. [DOI] [PubMed] [Google Scholar]
- Curwen K. D., Smith S. C. Aortic glycosaminoglycans in atherosclerosis-susceptible and -resistant pigeons. Exp Mol Pathol. 1977 Aug;27(1):121–133. doi: 10.1016/0014-4800(77)90024-7. [DOI] [PubMed] [Google Scholar]
- Dalferes E. R., Jr, Ruiz H., Kumar V., Radhakrishnamurthy B., Berenson G. S. Acid mucopolysaccharides of fatty streaks in young, human male aortas. Atherosclerosis. 1971 Jan-Feb;13(1):121–131. doi: 10.1016/0021-9150(71)90013-x. [DOI] [PubMed] [Google Scholar]
- Day A. J., Wahlqvist M. L. Cholesterol ester and phospholipid composition of normal aortas and of atherosclerotic lesions in children. Exp Mol Pathol. 1970 Oct;13(2):199–216. doi: 10.1016/0014-4800(70)90006-7. [DOI] [PubMed] [Google Scholar]
- Dayton S., Hashimoto S. Origin of fatty acids in lipids of experimental rabbit atheroma. J Atheroscler Res. 1968 May-Jun;8(3):555–568. doi: 10.1016/s0368-1319(68)80109-7. [DOI] [PubMed] [Google Scholar]
- Esterly J. A., Glagov S., Ferguson D. J. Morphogenesis of intimal obliterative hyperplasia of small arteries in experimental pulmonary hypertension. An ultrastructural study of the role of smooth-muscle cells. Am J Pathol. 1968 Feb;52(2):325–347. [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- GEER J. C., GUIDRY M. A. CHOLESTERYL ESTER COMPOSITION AND MORPHOLOGY OF HUMAN NORMAL INTIMA AND FATTY STREAKS. Exp Mol Pathol. 1964 Oct;90:485–499. doi: 10.1016/0014-4800(64)90029-2. [DOI] [PubMed] [Google Scholar]
- GOLDRICK B., HIRSCH J. A TECHNIQUE FOR QUANTITATIVE RECOVERY OF LIPIDS FROM CHROMATOPLATES. J Lipid Res. 1963 Oct;4:482–483. [PubMed] [Google Scholar]
- Geer J. C. Fine structure of human aortic intimal thickening and fatty streaks. Lab Invest. 1965 Oct;14(10):1764–1783. [PubMed] [Google Scholar]
- Haust M. D., More R. H., Bencosme S. A., Balis J. U. Electron microscopic studies in human atherosclerosis extracellular elements in aortic dots and streaks. Exp Mol Pathol. 1967 Jun;6(3):300–313. doi: 10.1016/0014-4800(67)90013-5. [DOI] [PubMed] [Google Scholar]
- Hoff H. F., Gaubatz J. W. Ultrastructural localization of plasma lipoproteins in human intracranial arteries. Virchows Arch A Pathol Anat Histol. 1975 Dec 31;369(2):111–121. doi: 10.1007/BF00433237. [DOI] [PubMed] [Google Scholar]
- Hojnacki J. L., Curwen K. D., Smith S. C. Cholesteryl esters of pigeon (Columba livia) aortas as a function of age. Comp Biochem Physiol B. 1977;57(1):19–22. doi: 10.1016/0305-0491(77)90075-x. [DOI] [PubMed] [Google Scholar]
- Hojnacki J. L., Smith S. C. Separation of six lipid classes on one thin-layer chromatogram. J Chromatogr. 1974 Apr 10;90(2):364–367. doi: 10.1016/s0021-9673(00)92542-1. [DOI] [PubMed] [Google Scholar]
- Hwang D. H., Mathias M. M., Dupont J., Meyer D. L. Linoleate enrichment of diet and prostaglandin metabolism in rats. J Nutr. 1975 Aug;105(8):995–1002. doi: 10.1093/jn/105.8.995. [DOI] [PubMed] [Google Scholar]
- Insull W., Jr, Bartsch G. E. Cholesterol, triglyceride, and phospholipid content of intima, media, and atherosclerotic fatty streak in human thoracic aorta. J Clin Invest. 1966 Apr;45(4):513–523. doi: 10.1172/JCI105365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. F., Jensen G. L., Smith S. C. Serum lipoprotein profiles of young atherosclerosis-susceptible White Carneau and atherosclerosis-resistant Show Racer pigeons. Comp Biochem Physiol B. 1978;60(1):67–69. doi: 10.1016/0305-0491(78)90029-9. [DOI] [PubMed] [Google Scholar]
- Jordan R. E., Hewitt N., Lewis W., Kagan H., Franzblau C. Regulation of elastase-catalyzed hydrolysis of insoluble elastin by synthetic and naturally occurring hydrophobic ligands. Biochemistry. 1974 Aug 13;13(17):3497–3503. doi: 10.1021/bi00714a013. [DOI] [PubMed] [Google Scholar]
- Joris I., Majno G. Cell-to-cell herniae in the arterial wall. I. The pathogenesis of vacuoles in the normal media. Am J Pathol. 1977 May;87(2):375–398. [PMC free article] [PubMed] [Google Scholar]
- Joris I., Majno G. Cellular breakdown within the arterial wall. An ultrastructural study of the coronary artery in young and aging rats. Virchows Arch A Pathol Anat Histol. 1974;364(1):111–127. doi: 10.1007/BF01230861. [DOI] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kalra V. K., Brodie A. F. Metabolic differences between the arteries of atheroscierotic susceptible and resistant pigeons. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1372–1378. doi: 10.1016/s0006-291x(74)80435-3. [DOI] [PubMed] [Google Scholar]
- Kalra V. K., Brodie A. F. The presence of the glycerol phosphate shuttle and energy dependent transhydrogenase in aortic mitochondria. Biochem Biophys Res Commun. 1973 Mar 17;51(2):414–420. doi: 10.1016/0006-291x(73)91273-4. [DOI] [PubMed] [Google Scholar]
- Kojimahara M., Ooneda G. Electron microscopic study on the middle cerebral artery lesions in hypertensive rats. Acta Pathol Jpn. 1970 Nov;20(4):399–408. doi: 10.1111/j.1440-1827.1970.tb00097.x. [DOI] [PubMed] [Google Scholar]
- Kritchevsky D., Kothari H. V. Aortic cholesterol esterase: studies in White Carneau and Show Racer pigeons. Biochim Biophys Acta. 1973 Dec 20;326(3):489–491. doi: 10.1016/0005-2760(73)90157-4. [DOI] [PubMed] [Google Scholar]
- LOFLAND H. B., Jr, MOURY D. M., HOFFMAN C. W., CLARKSON T. B. LIPID METABOLISM IN PIGEON AORTA DURING ATHEROGENESIS. J Lipid Res. 1965 Jan;6:112–118. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauper N. T., Unni K. K., Kottke B. A., Titus J. L. Anatomy and histology of aorta of White Carneau pigeon. Lab Invest. 1975 Apr;32(4):536–551. [PubMed] [Google Scholar]
- Lewis J. C., Kottke B. A. Endothelial damage and thrombocyte adhesion in pigeon atherosclerosis. Science. 1977 May 27;196(4293):1007–1009. doi: 10.1126/science.860128. [DOI] [PubMed] [Google Scholar]
- Moss N. S., Benditt E. P. The ultrastructure of spontaneous and experimentally induced arterial lesions. II. The spontaneous plaque in the chicken. Lab Invest. 1970 Sep;23(3):231–245. [PubMed] [Google Scholar]
- Nicolosi R. J., Santerre R. F., Smith S. C. Lipid accumulation in muscular foci in White Carneau and Show Racer pigeon aortas. Exp Mol Pathol. 1972 Aug;17(1):29–37. doi: 10.1016/0014-4800(72)90055-x. [DOI] [PubMed] [Google Scholar]
- Nicolosi R. J., Smith S. C., Santerre R. F. Simultaneous fluorometric analysis of five lipid classes on thin-layer chromatograms. J Chromatogr. 1971 Aug 5;60(1):111–117. [PubMed] [Google Scholar]
- PRICHARD R. W., CLARKSON T. B., LOFLAND H. B., Jr, GOODMAN H. O., HERNDON C. N., NETSKY M. G. Studies on the atherosclerotic pigeon. JAMA. 1962 Jan 6;179:49–52. doi: 10.1001/jama.1962.03050010000008d. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D. Cholesterol and cell membrane function: a hypothesis concerning etiology of atherosclerosis. J Theor Biol. 1974 Feb;43(2):329–337. doi: 10.1016/s0022-5193(74)80064-0. [DOI] [PubMed] [Google Scholar]
- Portman O. W., Alexander M., Maruffo C. A. Nutritional control of arterial lipid composition in squirrel monkeys: major ester classes and types of phospholipids. J Nutr. 1967 Jan;91(1):35–46. doi: 10.1093/jn/91.1.35. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTTER W., WELLMER H. K., HINRICHS G., MULLER W. Zur Orthologie und Pathologie der Polsterarterien (sog. Verzweigungs-und Spornpolster) des Gehirns. Beitr Pathol Anat. 1955;115(2):253–294. [PubMed] [Google Scholar]
- Ross R., Harker L. Hyperlipidemia and atherosclerosis. Science. 1976 Sep 17;193(4258):1094–1100. doi: 10.1126/science.822515. [DOI] [PubMed] [Google Scholar]
- Rymaszewski Z., Langelier M., Carlo I. A., Subbiah M. T., Kottke B. A. Age-related interrelationships of blood pressure and arterial sterol accumulation in spontaneously atherosclerosis-susceptible and atherosclerosis-resistant pigeons. Atherosclerosis. 1976 Jan-Feb;23(1):111–116. doi: 10.1016/0021-9150(76)90122-2. [DOI] [PubMed] [Google Scholar]
- SMITH S. C., STROUT R. G., DUNLOP W. R., SMITH E. C. FATTY ACID COMPOSITION OF CULTURED AORTIC CELLS FROM WHITE CARNEAU AND SHOW RACER PIGEONS. J Atheroscler Res. 1965 Jul-Aug;5(4):379–387. doi: 10.1016/s0368-1319(65)80073-4. [DOI] [PubMed] [Google Scholar]
- STEHBENS W. E. Focal intimal proliferation in the cerebral arteries. Am J Pathol. 1960 Mar;36:289–301. [PMC free article] [PubMed] [Google Scholar]
- STEHBENS W. E., PHIL D. THE RENAL ARTERY IN NORMAL AND CHOLESTEROL-FED RABBITS. Am J Pathol. 1963 Dec;43:969–985. [PMC free article] [PubMed] [Google Scholar]
- Salgado E. D. Medial aortic lesions in rats with metacorticoid hypertension. Am J Pathol. 1970 Feb;58:305–327. [PMC free article] [PubMed] [Google Scholar]
- Santerre R. F., Nicolosi R. J., Smith S. C. Respiratory control in preatherosclerotic susceptible and resistant pigeon aortas. Exp Mol Pathol. 1974 Jun;20(3):397–406. doi: 10.1016/0014-4800(74)90069-0. [DOI] [PubMed] [Google Scholar]
- Santerre R. F., Wight T. N., Smith S. C., Brannigan D. Spontaneous atherosclerosis in pigeons. A model system for studying metabolic parameters associated with atherogenesis. Am J Pathol. 1972 Apr;67(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- Scott R. F., Jones R., Daoud A. S., Zumbo O., Coulston F., Thomas W. A. Experimental atherosclerosis in rhesus monkeys. II. Cellular elements of proliferative lesions and possible role of cytoplasmic degeneration in pathogenesis as studied by electron microscopy. Exp Mol Pathol. 1967 Aug;7(1):34–57. doi: 10.1016/0014-4800(67)90037-8. [DOI] [PubMed] [Google Scholar]
- Smith E. B., Evans P. H., Downham M. D. Lipid in the aortic intima. The correlation of morphological and chemical characteristics. J Atheroscler Res. 1967 Mar-Apr;7(2):171–186. doi: 10.1016/s0368-1319(67)80079-6. [DOI] [PubMed] [Google Scholar]
- St Clair R. W., Lofland H. B., Clarkson T. B. Influence of duration of cholesterol feeding on esterification of fatty acids by cell-free preparation of pigeon aorta. Circ Res. 1970 Aug;27(2):213–225. doi: 10.1161/01.res.27.2.213. [DOI] [PubMed] [Google Scholar]
- St Clair R. W., Lofland H. B., Jr, Clarkson T. B. Composition and synthesis of fatty acids in atherosclerotic aortas of the pigeon. J Lipid Res. 1968 Nov;9(6):739–747. [PubMed] [Google Scholar]
- Stary H. C. Coronary artery fine structure in rhesus monkeys: the early atherosclerotic lesion and its progression. Primates Med. 1976;9:359–395. [PubMed] [Google Scholar]
- Stehbens W. E. Cerebral atherosclerosis. Intimal proliferation and atherosclerosis in the cerebral arteries. Arch Pathol. 1975 Nov;99(11):582–591. [PubMed] [Google Scholar]
- Stehbens W. E. Intimal proliferation and spontaneous lipid deposition in the cerebral arteies of sheep and steers. J Atheroscler Res. 1965 Nov-Dec;5(6):556–568. doi: 10.1016/s0368-1319(65)80032-1. [DOI] [PubMed] [Google Scholar]
- Stehbens W. E., Ludatscher R. M. Ultrastructure of the renal arterial bifurcation of rabbits. Exp Mol Pathol. 1973 Feb;18(1):50–67. doi: 10.1016/0014-4800(73)90007-5. [DOI] [PubMed] [Google Scholar]
- Subbiah M. T., Dicke B. A. On the cholesteryl ester hydrolase activity in the microsomal and supernatant fractions of pigeon aorta. Atherosclerosis. 1976 Sep;24(3):575–580. doi: 10.1016/0021-9150(76)90149-0. [DOI] [PubMed] [Google Scholar]
- Subbiah M. T., Unni K. K., Kottke B. A., Carlo I. A., Dinh D. M. Arterial and metabolic changes during the critical period of spontaneous sterol accumulation in pigeon aorta. Exp Mol Pathol. 1976 Jun;24(3):287–301. doi: 10.1016/0014-4800(76)90066-6. [DOI] [PubMed] [Google Scholar]
- Takebayashi S. Ultrastructural studies on arteriolar lesions in experimental hypertension. J Electron Microsc (Tokyo) 1970;19(1):17–31. [PubMed] [Google Scholar]
- WILENS S. L. The nature of diffuse intimal thickening of arteries. Am J Pathol. 1951 Sep-Oct;27(5):825–839. [PMC free article] [PubMed] [Google Scholar]
- Wagner W. D., Clarkson T. B., Feldner M. A., Prichard R. W. The development of pigeon strains with selected atherosclerosis characteristics. Exp Mol Pathol. 1973 Dec;19(3):304–319. doi: 10.1016/0014-4800(73)90062-2. [DOI] [PubMed] [Google Scholar]
- Whereat A. F. Is atherosclerosis a disorder of intramitochondrial respiration? Ann Intern Med. 1970 Jul;73(1):125–127. doi: 10.7326/0003-4819-73-1-125. [DOI] [PubMed] [Google Scholar]
- Wiener J., Giacomelli F. The cellular pathology of experimental hypertension. 7. Structure and permeability of the mesenteric vasculature in angiotensin-induced hypertension. Am J Pathol. 1973 Aug;72(2):221–231. [PMC free article] [PubMed] [Google Scholar]
- Wojtczak L. Effect of long-chain fatty acids and acyl-CoA on mitochondrial permeability, transport, and energy-coupling processes. J Bioenerg Biomembr. 1976 Dec;8(6):293–311. doi: 10.1007/BF00765158. [DOI] [PubMed] [Google Scholar]
- Zemplenyi T., Rosenstein A. J. Arterial enzymes and their relation to atherosclerosis in pigeons. Exp Mol Pathol. 1975 Apr;22(2):225–241. doi: 10.1016/0014-4800(75)90066-0. [DOI] [PubMed] [Google Scholar]








