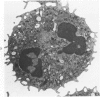
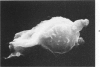
Abstract
1-Palmitoyl-lysophosphatidic acid (LPA) was studied for its influence on the chemotaxis and ultrastructure of human neutrophils. By itself, LPA had no effect on the indices of chemotaxis or random migration of neutrophils. However, LPA on either the cellular or attractant side of Boyden chambers significantly enhanced the chemotactic responses of neutrophils to suboptimal concentrations of formyl-methionyl-phenylalanine. The enhancement of chemotaxis was achieved with concentrations of LPA (120-240 microM) that had no effect alone on neutrophil ultrastructure. The results, taken together with recent advances in knowledge of the role of the phosphatidylinositol turnover response in mediating effects of stimulating agents on cells, may provide a novel concept for understanding neutrophil chemotaxis.
Full text
PDF


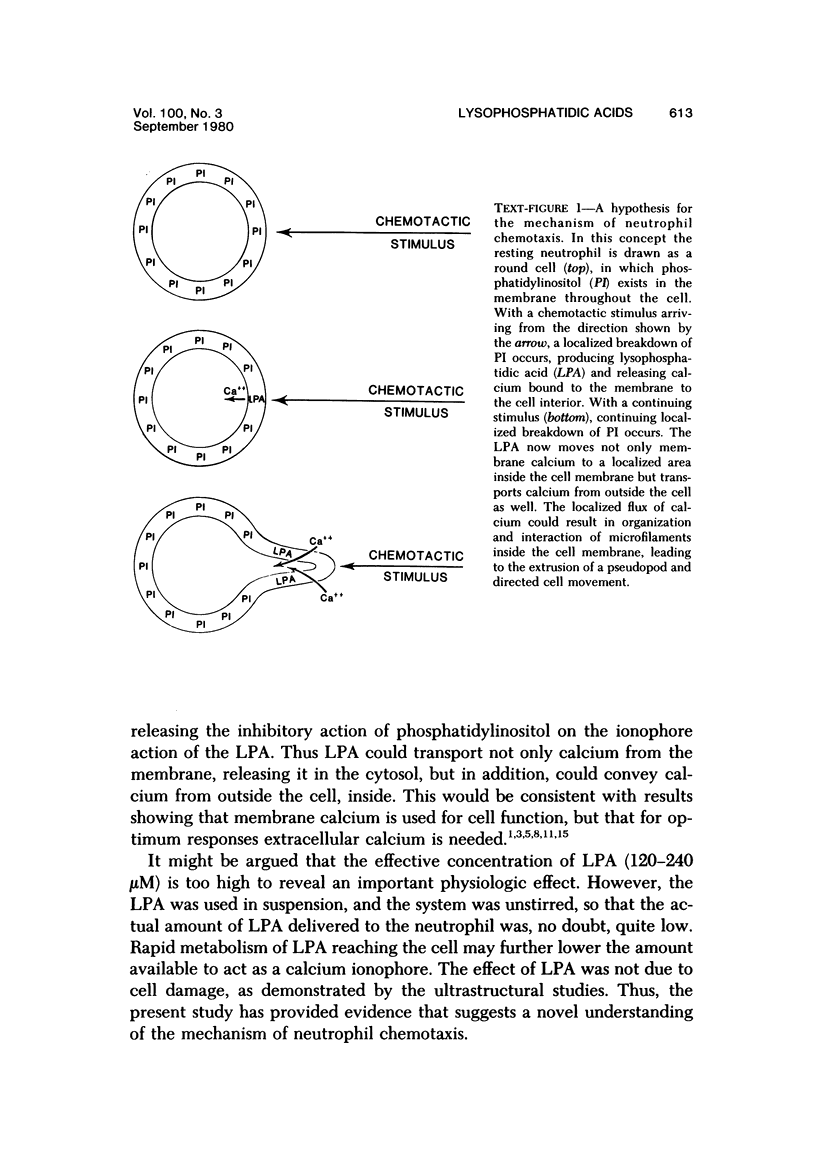



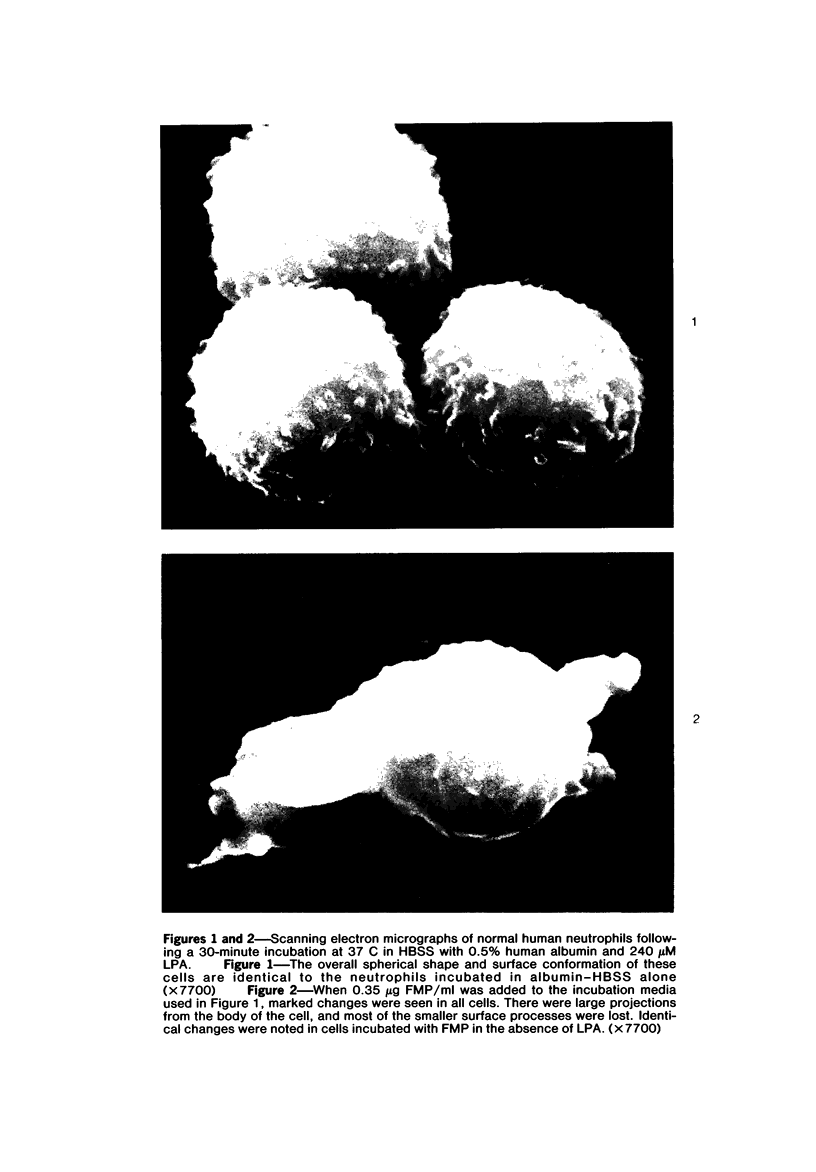
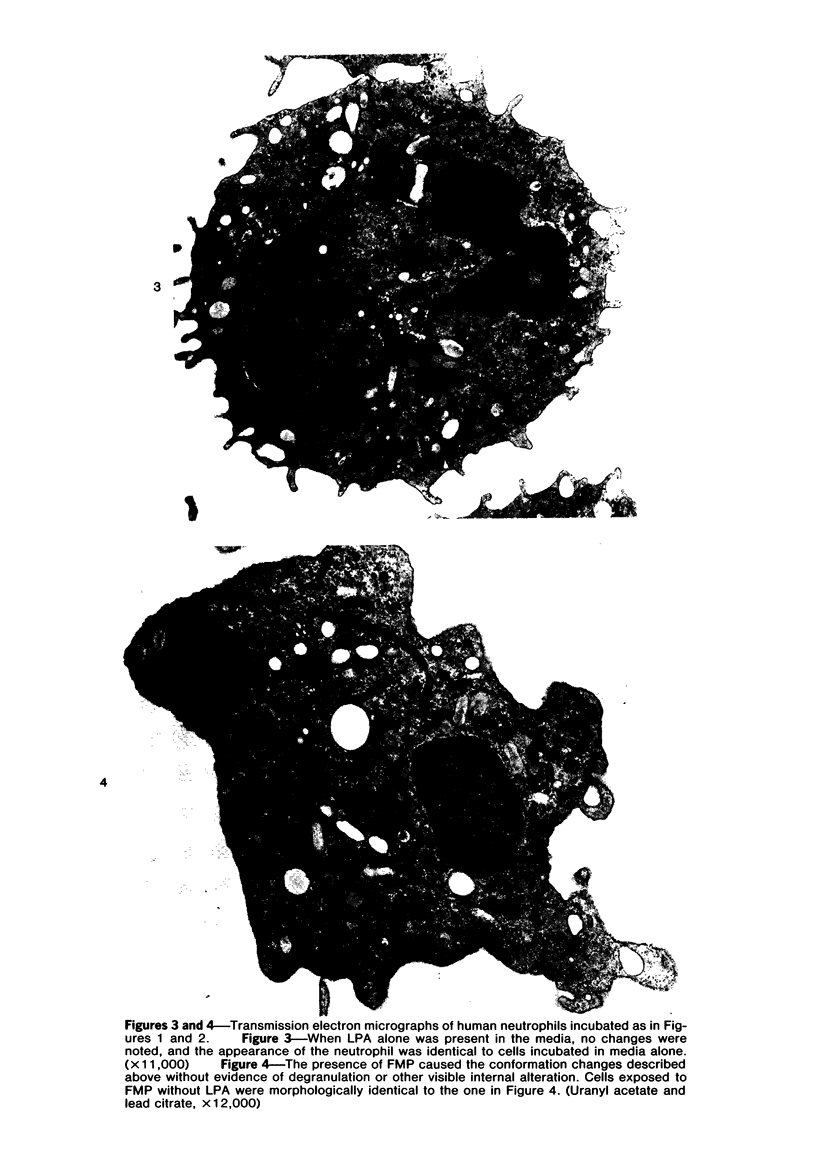
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker E. L., Showell H. J. The effect of Ca2+ and Mg2+ on the chemotactic responsiveness and spontaneous motility of rabbit polymorphonuclear leukocytes. Z Immunitatsforsch Exp Klin Immunol. 1972 Jun;143(5):466–476. [PubMed] [Google Scholar]
- Berridge M. J., Fain J. N. Inhibition of phosphatidylinositol synthesis and the inactivation of calcium entry after prolonged exposure of the blowfly salivary gland to 5-hydroxytryptamine. Biochem J. 1979 Jan 15;178(1):59–69. doi: 10.1042/bj1780059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch G. M., Reed P. W. Inhibition of the neutrophil oxidative response to a chemotactic peptide by inhibitors of arachidonic acid oxygenation. Biochem Biophys Res Commun. 1979 Sep 27;90(2):481–487. doi: 10.1016/0006-291x(79)91260-9. [DOI] [PubMed] [Google Scholar]
- Boucek M. M., Snyderman R. Calcium influx requirement for human neutrophil chemotaxis: inhibition by lanthanum chloride. Science. 1976 Sep 3;193(4256):905–907. doi: 10.1126/science.948752. [DOI] [PubMed] [Google Scholar]
- Cramer E. B., Gallin J. I. Localization of submembranous cations to the leading end of human neutrophils during chemotaxis. J Cell Biol. 1979 Aug;82(2):369–379. doi: 10.1083/jcb.82.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estensen R. D., Reusch M. E., Epstein M. L., Hill H. R. Role of Ca2+ and Mg2+ in some human neutrophil functions as indicated by ionophore A23187. Infect Immun. 1976 Jan;13(1):146–151. doi: 10.1128/iai.13.1.146-151.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain J. N., Berridge M. J. Relationship between hormonal activation of phosphatidylinositol hydrolysis, fluid secretion and calcium flux in the blowfly salivary gland. Biochem J. 1979 Jan 15;178(1):45–58. doi: 10.1042/bj1780045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Rosenthal A. S. The regulatory role of divalent cations in human granulocyte chemotaxis. Evidence for an association between calcium exchanges and microtubule assembly. J Cell Biol. 1974 Sep;62(3):594–609. doi: 10.1083/jcb.62.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard J. M., Butler A. M., Peterson D. A., White J. G. Phosphatidic acid releases calcium from a platelet membrane fraction in vitro. Prostaglandins Med. 1978 Nov;1(5):387–396. doi: 10.1016/0161-4630(78)90125-8. [DOI] [PubMed] [Google Scholar]
- Gerrard J. M., Kindom S. E., Peterson D. A., Peller J., Krantz K. E., White J. G. Lysophosphatidic acids. Influence on platelet aggregation and intracellular calcium flux. Am J Pathol. 1979 Aug;96(2):423–438. [PMC free article] [PubMed] [Google Scholar]
- Gerrard J. M., Kindom S. E., Peterson D. A., White J. G. Lysophosphatidic acids. II. Interaction of the effects of adenosine diphosphate and lysophosphatidic acids in dog, rabbit, and human platelets. Am J Pathol. 1979 Dec;97(3):531–547. [PMC free article] [PubMed] [Google Scholar]
- Gerrard J. M., White J. G. Prostaglandins and thromboxanes: "middlemen" modulating platelet function in hemostasis and thrombosis. Prog Hemost Thromb. 1978;4:87–125. [PubMed] [Google Scholar]
- Hawthorne J. N., White D. A. Myo-inositol lipids. Vitam Horm. 1975;33:529–573. doi: 10.1016/s0083-6729(08)60972-3. [DOI] [PubMed] [Google Scholar]
- Hoffstein S. T. Ultrastructural demonstration of calcium loss from local regions of the plasma membrane of surface-stimulated human granulocytes. J Immunol. 1979 Sep;123(3):1395–1402. [PubMed] [Google Scholar]
- KARNOVSKY M. L., WALLACH D. F. The metabolic basis of phagocytosis. III. Incorporation of inorganic phosphate into various classes of phosphatides during phagocytosis. J Biol Chem. 1961 Jul;236:1895–1901. [PubMed] [Google Scholar]
- Lapetina E. G., Michell R. H. Phosphatidylinositol metabolism in cells receiving extracellular stimulation. FEBS Lett. 1973 Apr 1;31(1):1–10. doi: 10.1016/0014-5793(73)80061-4. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Miller J. C., Leung I. The effects of barbiturates on the metabolism of phosphatidic acid and phosphatidylinositol in rat brain synaptosomes. Biochem J. 1979 Jan 15;178(1):9–13. doi: 10.1042/bj1780009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Changes in ionic movements across rabbit polymorphonuclear leukocyte membranes during lysosomal enzyme release. Possible ionic basis for lysosomal enzyme release. J Cell Biol. 1977 Dec;75(3):635–649. doi: 10.1083/jcb.75.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Involvement of membrane calcium in the response of rabbit neutrophils to chemotactic factors as evidenced by the fluorescence of chlorotetracycline. J Cell Biol. 1979 Oct;83(1):179–186. doi: 10.1083/jcb.83.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Transport of sodium, potassium, and calcium across rabbit polymorphonuclear leukocyte membranes. Effect of chemotactic factor. J Cell Biol. 1977 May;73(2):428–444. doi: 10.1083/jcb.73.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Volpi M., Showell H. J., Becker E. L., Sha'afi R. I. Chemotactic factor-induced release of membrane calcium in rabbit neutrophils. Science. 1979 Feb 2;203(4379):461–463. doi: 10.1126/science.760200. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. J., Becker E. L., Ward P. A. Role of arachidonic acid derivatives in neutrophil aggregation: a hypothesis. Prostaglandins. 1979 Jun;17(6):915–927. doi: 10.1016/0090-6980(79)90062-5. [DOI] [PubMed] [Google Scholar]
- Oliver J. M., Krawiec J. A., Becker E. L. The distribution of actin during chemotaxis in rabbit neutrophils. J Reticuloendothel Soc. 1978 Dec;24(6):697–704. [PubMed] [Google Scholar]
- Petroski R. J., Naccache P. H., Becker E. L., Sha'afi R. I. Effect of the chemotactic factor formyl methionyl- leucyl-phenylalanine and cytochalasin B on the cellular levels of calcium in rabbit neutrophils. FEBS Lett. 1979 Apr 1;100(1):161–165. doi: 10.1016/0014-5793(79)81155-2. [DOI] [PubMed] [Google Scholar]
- Repine J. E., Clawson C. C. Influence of surface proteins and separation techniques on neutrophil unstimulated and stimulated locomotion in vitro. J Reticuloendothel Soc. 1978 Sep;24(3):217–226. [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. 2. Incorporation of C14-labeled building blocks into lipid, protein, and glycogen of leukocytes during phagocytosis. J Biol Chem. 1960 Aug;235:2224–2229. [PubMed] [Google Scholar]
- Sastry P. S., Hokin L. E. Studies on the role of phospholipids in phagocytosis. J Biol Chem. 1966 Jul 25;241(14):3354–3361. [PubMed] [Google Scholar]
- Senda N., Shibata N., Tamura H., Yoshitake J. Leucocytic movement and contractile protein. Methods Achiev Exp Pathol. 1979;9:169–186. [PubMed] [Google Scholar]
- Senda N., Tamura H., Shibata N., Yoshitake J., Konko K., Tanaka K. The mechanism of the movement of leucocytes. Exp Cell Res. 1975 Mar 15;91(2):393–407. doi: 10.1016/0014-4827(75)90120-2. [DOI] [PubMed] [Google Scholar]
- Shibata N., Tatsumi N., Tanaka K., Okamura Y., Senda N. A contractile protein possessing Ca 2+ sensitivity (natural actomyosin) from leucocytes. Its extraction and some of its properties. Biochim Biophys Acta. 1972 Feb 28;256(2):565–576. doi: 10.1016/0005-2728(72)90084-9. [DOI] [PubMed] [Google Scholar]
- Showell H. J., Naccache P. H., Sha'afi R. I., Becker E. L. The effects of extracellular K+, Na+ and Ca++ on lysosomal enzyme secretion from polymorphonuclear leukocytes. J Immunol. 1977 Sep;119(3):804–811. [PubMed] [Google Scholar]
- Smith R. J., Ignarro L. J. Bioregulation of lysosomal enzyme secretion from human neutrophils: roles of guanosine 3':5'-monophosphate and calcium in stimulus-secretion coupling. Proc Natl Acad Sci U S A. 1975 Jan;72(1):108–112. doi: 10.1073/pnas.72.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tou J. S. Fatty acid and glycerol labeling of glycerolipids of leukocytes in response to ionophore A23187. Biochim Biophys Acta. 1979 Feb 26;572(2):307–313. doi: 10.1016/0005-2760(79)90046-8. [DOI] [PubMed] [Google Scholar]
- Tou J. S., Maier C. Phospholipid metabolism and lysosomal enzyme secretion by leukocytes. Effects of dibutyryl cyclic adenosine 3':5'-monophosphate and ATP. Biochim Biophys Acta. 1976 Dec 21;451(2):353–362. doi: 10.1016/0304-4165(76)90130-6. [DOI] [PubMed] [Google Scholar]
- Tou J. S. Modulation of 32Pi incorporation into phospholipids of polymorphonuclear leukocytes by ionophore A23187. Biochim Biophys Acta. 1978 Nov 22;531(2):167–178. doi: 10.1016/0005-2760(78)90140-6. [DOI] [PubMed] [Google Scholar]
- Tou J. S., Stjernholm R. L. Stimulation of the incorporation of 32Pi and myo-(2-3H)inositol into the phosphoinositides in polymorphonuclear leukocytes during phagocytosis. Arch Biochem Biophys. 1974 Feb;160(2):487–494. doi: 10.1016/0003-9861(74)90425-1. [DOI] [PubMed] [Google Scholar]
- Tou K. J., Stjernholm R. L. Cytochalasin B: effect on phospholipid metabolism and lysosomal enzyme release by leukocytes;. Biochim Biophys Acta. 1975 May 5;392(1):1–11. doi: 10.1016/0304-4165(75)90160-9. [DOI] [PubMed] [Google Scholar]
- Tyson C. A., Vande Zande H., Green D. E. Phospholipids as ionophores. J Biol Chem. 1976 Mar 10;251(5):1326–1332. [PubMed] [Google Scholar]
- WOODIN A. M., WIENEKE A. A. The incorporation of radioactive phosphorus in the leucocyte during the extrusion of protein induced by staphylococcal leucocidin. Biochem J. 1963 Jun;87:480–487. doi: 10.1042/bj0870480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Korchak H. M., Perez H. D., Smolen J. E., Goldstein I. M., Hoffstein S. T. The secretory code of the neutrophil. J Reticuloendothel Soc. 1979 Dec;26(Suppl):687–700. [PubMed] [Google Scholar]
- Wilkinson P. C. Leucocyte locomotion and chemotaxis. The influence of divalent cations and cation ionophores. Exp Cell Res. 1975 Jul;93(2):420–426. doi: 10.1016/0014-4827(75)90468-1. [DOI] [PubMed] [Google Scholar]