Abstract
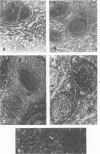
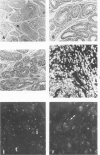
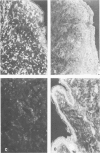
Inoculation of the causative agent of syphilis, Treponema pallidum into the testes of rabbits initiated the following sequence of events: 1) a rapid proliferation of organisms in the interstitial tissues of the testes, reaching a maximum at about 10-11 days after infection; 2) systemic spread of organisms primarily in the lymphoid organs; 3) a prompt immune response manifested by hyperplasia of T cell domains in draining lymph nodes and spleen, blast transformation responses of lymphoid cells to sonicates of T pallidum, the appearance of serum antibody, and the marked infiltration of the infected areas of the testes by T cells; 4) essential clearing of organisms identified by immunofluorescence from the infected site 10-14 days after infection associated with evolution of the inflammatory response from primarily a T cell infiltrate to a larger mononuclear cell type, and the immunofluorescent identification of presumptive T pallidum antigen in macrophages; 5) interstitial fibrosis or resolution 17-21 days after infection so that examination of infected testes from 1 to 24 months later reveals foci of tubular atrophy and fibrosis of varying size, alternating with regenerated tubules, separated by interstitial areas with only minimal fibrosis. During the long period of latency there is no evidence of atrophy or hypoplasia of the lymphoid organs and long-lasting T cell memory with regard to T pallidum sonicates is demonstrable. Reinfection of previously inoculated rabbits indicates partial protection at 25 days after infection followed by essentially complete protection after 55 days. It is concluded that there is a prompt and long-lasting immune response to T pallidum in experimentally infected rabbits. The main mechanism for destruction of infecting organisms appears to be T-cell-initiated macrophage-mediated destruction, but a role for antibody dependent phagocytosis cannot be ruled out. The reason that some organisms may survive in various body organs remains unknown, but possible explanations are presented.
Full text
PDF



























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baughn R. E., Musher D. M. Aberrant secondary antibody responses to sheep erythrocytes in rabbits with experimental syphilis. Infect Immun. 1979 Jul;25(1):133–138. doi: 10.1128/iai.25.1.133-138.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn R. E., Musher D. M. Altered immune responsiveness associated with experimental syphilis in the rabbit: elevated IgM and depressed IgG responses to sheep erythrocytes. J Immunol. 1978 May;120(5):1691–1695. [PubMed] [Google Scholar]
- Bey R. F., Johnson R. C., Fitzgerald T. J. Suppression of lymphocyte response to concanavalin A by mucopolysaccharide material from Treponema pallidum-infected rabbits. Infect Immun. 1979 Oct;26(1):64–69. doi: 10.1128/iai.26.1.64-69.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop N. H., Miller J. N. Humoral immunity in experimental syphilis. I. The demonstration of resistance conferred by passive immunization. J Immunol. 1976 Jul;117(1):191–196. [PubMed] [Google Scholar]
- Bishop N. H., Miller J. N. Humoral immunity in experimental syphilis. II. The relationship of neutralizing factors in immune serum to acquired resistance. J Immunol. 1976 Jul;117(1):197–207. [PubMed] [Google Scholar]
- Bloom B. R. Games parasites play: how parasites evade immune surveillance. Nature. 1979 May 3;279(5708):21–26. doi: 10.1038/279021a0. [DOI] [PubMed] [Google Scholar]
- Brause B. D., Roberts R. B. Attachment of virulent Treponema pallidum to human mononuclear phagocytes. Br J Vener Dis. 1978 Aug;54(4):218–224. doi: 10.1136/sti.54.4.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTIANSEN S. Protective layer covering pathogenic treponemata. Lancet. 1963 Feb 23;1(7278):423–425. doi: 10.1016/s0140-6736(63)92309-2. [DOI] [PubMed] [Google Scholar]
- FRAZIER C. N., BENSEL A., KEUPER C. S. Further observations on the duration of spirochetemia in rabbits with asymptomatic syphilis. Am J Syph Gonorrhea Vener Dis. 1952 Mar;36(2):167–173. [PubMed] [Google Scholar]
- Festenstein H., Abrahams C., Bokkenheuser V. Runting syndrome in neonatal rabbits infected with Treponema pallidum. Clin Exp Immunol. 1967 May;2(3):311–320. [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C. Surface mucopolysaccharides of Treponema pallidum. Infect Immun. 1979 Apr;24(1):244–251. doi: 10.1128/iai.24.1.244-251.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann P. S., Turk J. L. The role of cell-mediated immune mechanisms in syphilis in Ethiopia. Clin Exp Immunol. 1978 Jan;31(1):59–65. [PMC free article] [PubMed] [Google Scholar]
- From E., Thestrup-Pedersen K., Thulin H. Reactivity of lymphocytes from patients with syphilis towards T. pallidum antigen in the leucocyte migration and lymphocyte transformation tests. Br J Vener Dis. 1976 Aug;52(4):224–229. doi: 10.1136/sti.52.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves S., Alden J. Limited protection of rabbits against infection with Treponema pallidum by immune rabbit sera. Br J Vener Dis. 1979 Dec;55(6):399–403. doi: 10.1136/sti.55.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Frasch A. C., Bernards A., Borst P., Cross G. A. Novel expression-linked copies of the genes for variant surface antigens in trypanosomes. Nature. 1980 Mar 6;284(5751):78–80. doi: 10.1038/284078a0. [DOI] [PubMed] [Google Scholar]
- Horikawa M., Chisaka N., Yokoyama S., Onoé T. Effect of stirring during fixation upon immunofluorescence. Results with distribution of albumin-producing cells in liver. J Histochem Cytochem. 1976 Aug;24(8):926–932. doi: 10.1177/24.8.60440. [DOI] [PubMed] [Google Scholar]
- Levene G. M., Turk J. L., Wright D. J., Grimble A. G. Reduced lymphocyte transformation due to a plasma factor in patients with active syphilis. Lancet. 1969 Aug 2;2(7614):246–247. doi: 10.1016/s0140-6736(69)90010-5. [DOI] [PubMed] [Google Scholar]
- Levene G. M., Wright D. J., Turk J. L. Cell-mediated immunity and lymphocyte transformation in syphilis. Proc R Soc Med. 1971 Apr;64(4):426–428. doi: 10.1177/003591577106400440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Lloyd R. M., Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J Immunol. 1980 Jan;124(1):461–467. [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. I. In vitro response to mitogens and Treponema pallidum antigens. J Immunol. 1980 Jan;124(1):454–460. [PubMed] [Google Scholar]
- Lukehart S. A., Miller J. N. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J Immunol. 1978 Nov;121(5):2014–2024. [PubMed] [Google Scholar]
- MILLER J. N. THE APPEARANCE AND PERSISTENCE OF VDRL, RPCF, AND TPI ANTIBODY DURING THE COURSE AND TREATMENT OF EXPERIMENTAL SYPHILIS IN THE RABBIT. J Invest Dermatol. 1964 May;42:367–371. doi: 10.1038/jid.1964.80. [DOI] [PubMed] [Google Scholar]
- Maret S. M., Baseman J. B., Folds J. D. Cell-mediated immunity in Treponema pallidum infected rabbits: in vitro response of splenic and lymph node lymphocytes to mitogens and specific antigens. Clin Exp Immunol. 1980 Jan;39(1):38–43. [PMC free article] [PubMed] [Google Scholar]
- Medici M. A. The immunoprotective niche--a new pathogenic mechanism for syphilis, the systemic mycoses and other infectious diseases. J Theor Biol. 1972 Sep;36(3):617–625. doi: 10.1016/0022-5193(72)90012-4. [DOI] [PubMed] [Google Scholar]
- Musher D. M., Schell R. F., Jones R. H., Jones A. M. Lymphocyte transformation in syphilis: an in vitro correlate of immune suppression in vivo? Infect Immun. 1975 Jun;11(6):1261–1264. doi: 10.1128/iai.11.6.1261-1264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musher D. M., Schell R. F., Knox J. M. In vitro lymphocyte response to Treponema refringens im human syphilis. Infect Immun. 1974 Apr;9(4):654–657. doi: 10.1128/iai.9.4.654-657.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Baseman J. B., Folds J. D. Selective response of lymphocytes from Treponema pallidum-infected rabbits to mitogens and Treponema reiteri. Infect Immun. 1977 Feb;15(2):417–422. doi: 10.1128/iai.15.2.417-422.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Folds J. D., Baseman J. B. Depression of lymphocyte response to concanavalin A in rabbits infected with Treponema pallidum (Nichols strain). Infect Immun. 1976 Jul;14(1):320–322. doi: 10.1128/iai.14.1.320-322.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Folds J. D., Baseman J. B. Depression of lymphocyte response to concanavalin A in rabbits infected with Treponema pallidum (Nichols strain). Infect Immun. 1976 Jul;14(1):320–322. doi: 10.1128/iai.14.1.320-322.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Folds J. D., Baseman J. B. Development of of macrophage migration inhibition in rabbits infected with virulent Treponema pallidum. Infect Immun. 1977 Sep;17(3):651–654. doi: 10.1128/iai.17.3.651-654.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Folds J. D., Baseman J. B. Selective in vitro response of thymus-derived lymphocytes from Treponema pallidum-infected rabbits. Infect Immun. 1977 Dec;18(3):603–611. doi: 10.1128/iai.18.3.603-611.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavis C. S., Folds J. D., Baseman J. B. Cell-mediated immunity during syphilis. Br J Vener Dis. 1978 Jun;54(3):144–150. doi: 10.1136/sti.54.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perine P. L., Weiser R. S., Klebanoff S. J. Immunity to syphilis. I. Passive transfer in rabbits with hyperimmune serum. Infect Immun. 1973 Nov;8(5):787–790. doi: 10.1128/iai.8.5.787-790.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S. Distribution of alpha-fetoprotein- and albumin-containing cells in the livers of Fischer rats fed four cycles of N-2-fluorenylacetamide. Cancer Res. 1978 Sep;38(9):3107–3113. [PubMed] [Google Scholar]
- Shannon R., Booth S. D. The pattern of immunological responses at various stages of syphilis. Br J Vener Dis. 1977 Oct;53(5):281–286. doi: 10.1136/sti.53.5.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard H. W., Jr, Redelman D., Sell S. In vitro studies of the rabbit immune system. IV. Differential mitogen responses of isolated T and B cells. Cell Immunol. 1976 Jun 1;24(1):34–44. doi: 10.1016/0008-8749(76)90129-5. [DOI] [PubMed] [Google Scholar]
- THIVOLET J., SIMERAY A., ROLLAND M., CHALLUT F. Etude de l'intradermoréaction aux suspensions de Tréponèmes formolées souche Nichols pathogène chez les syphilitiques et les sujets normaux. Ann Inst Pasteur (Paris) 1953 Jul;85(1):23–33. [PubMed] [Google Scholar]
- Titus R. G., Weiser R. S. Experimental syphilis in the rabbit: passive transfer of immunity with immunoglobulin G from immune serum. J Infect Dis. 1979 Dec;140(6):904–913. doi: 10.1093/infdis/140.6.904. [DOI] [PubMed] [Google Scholar]
- Ware J. L., Folds J. D., Baseman J. B. Serum of rabbits infected with Treponema pallidum (Nichols) inhibits in vitro transformation of normal rabbit lymphocytes. Cell Immunol. 1979 Feb;42(2):363–372. doi: 10.1016/0008-8749(79)90201-6. [DOI] [PubMed] [Google Scholar]
- Wicher V., Wicher K. Cell response in rabbits infected with T. pallidum as measured by the leucocyte migration inhibition test. Br J Vener Dis. 1975 Aug;51(4):240–245. doi: 10.1136/sti.51.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher V., Wicher K. In vitro cell response of Treponema pallidum-infected rabbits. I. Lymphocyte transformation. Clin Exp Immunol. 1977 Sep;29(3):480–486. [PMC free article] [PubMed] [Google Scholar]
- Wicher V., Wicher K. In vitro cell response of Treponema pallidum-infected rabbits. II. Inhibition of lymphocyte response to phytohaemagglutinin by serum of T. pallidum-infected rabbits. Clin Exp Immunol. 1977 Sep;29(3):487–495. [PMC free article] [PubMed] [Google Scholar]
- Wicher V., Wicher K. In vitro cell response of Treponema pallidum-infected rabbits. III. Impairment in production of lymphocyte mitogenic factor. Clin Exp Immunol. 1977 Sep;29(3):496–500. [PMC free article] [PubMed] [Google Scholar]
- Williams R. O., Young J. R., Majiwa P. A. Genomic rearrangements correlated with antigenic variation in Trypanosoma brucei. Nature. 1979 Dec 20;282(5741):847–849. doi: 10.1038/282847a0. [DOI] [PubMed] [Google Scholar]
- Wright D. J., Grimble A. S. Why is the infectious stage of syphilis prolonged? Br J Vener Dis. 1974 Feb;50(1):45–49. doi: 10.1136/sti.50.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H., Shortman K. The separation of different cell classes from lymphoid organs. IX. A simple and rapid method for removal of damaged cells from lymphoid cell suspensions. J Immunol Methods. 1973 Apr;2(3):293–301. doi: 10.1016/0022-1759(73)90055-0. [DOI] [PubMed] [Google Scholar]