Abstract
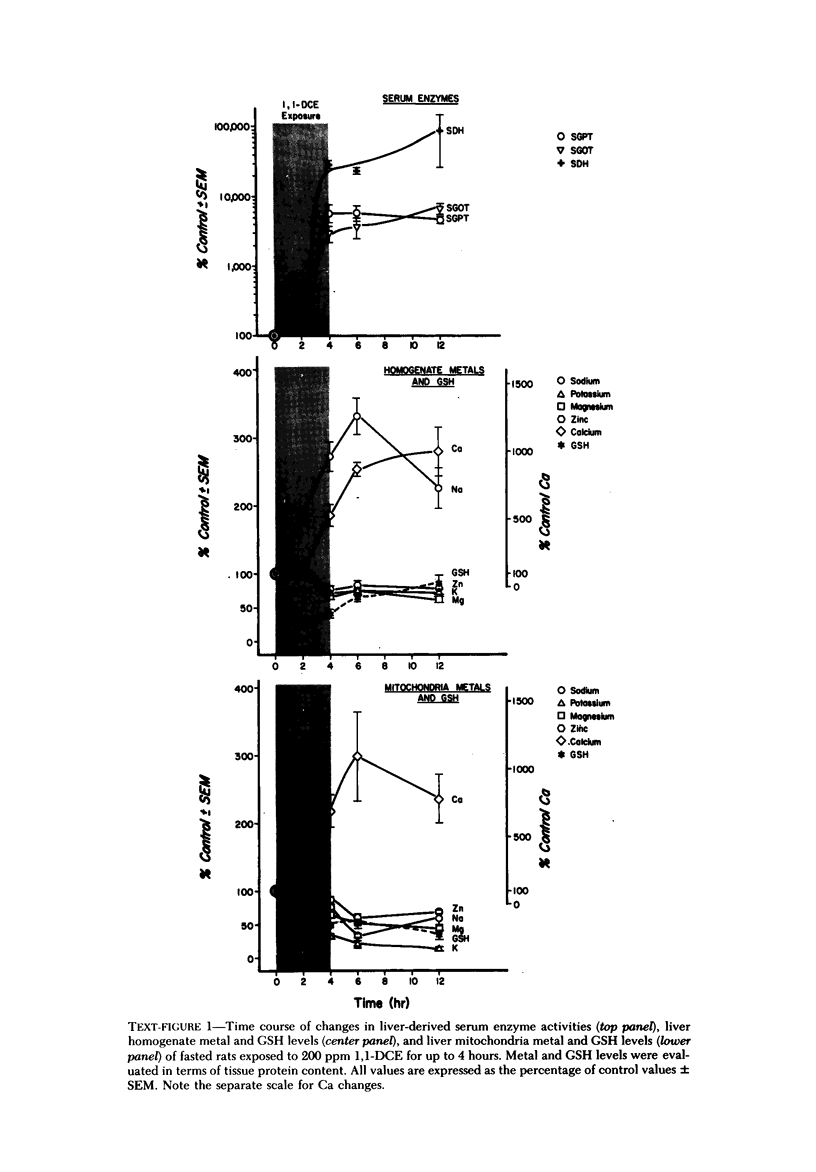
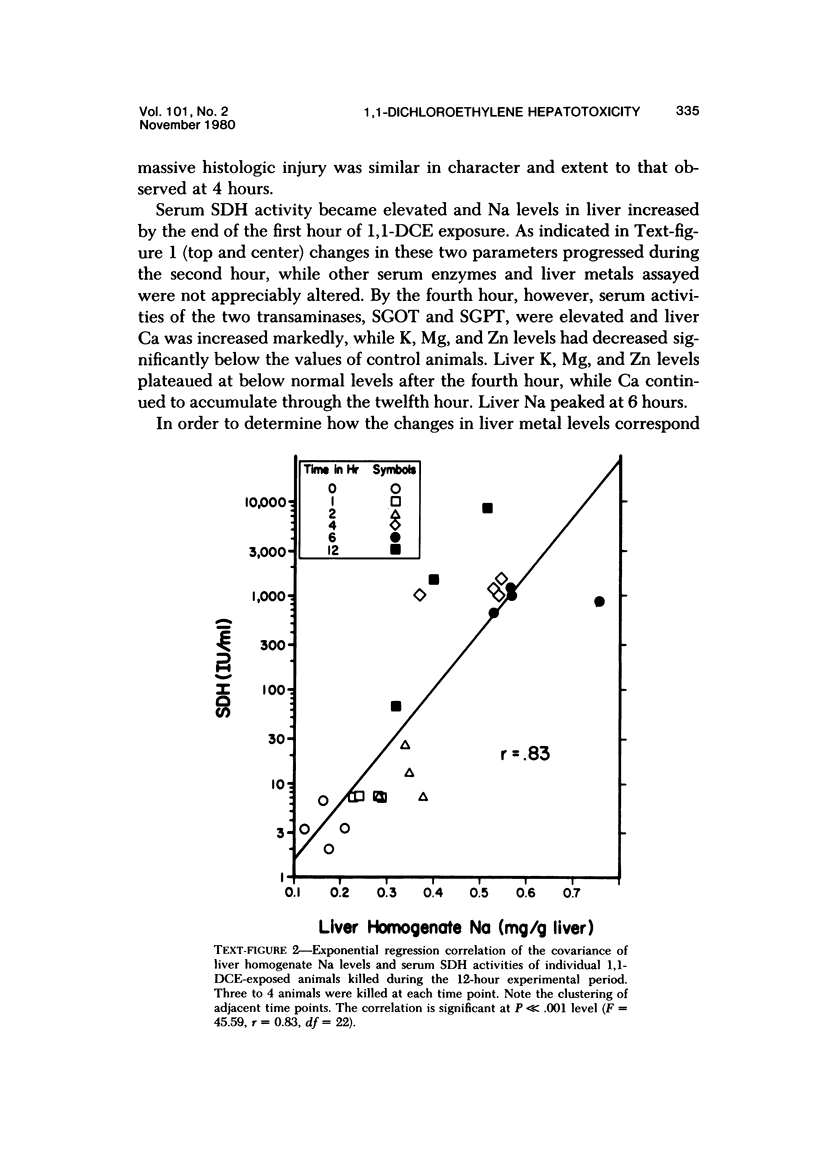
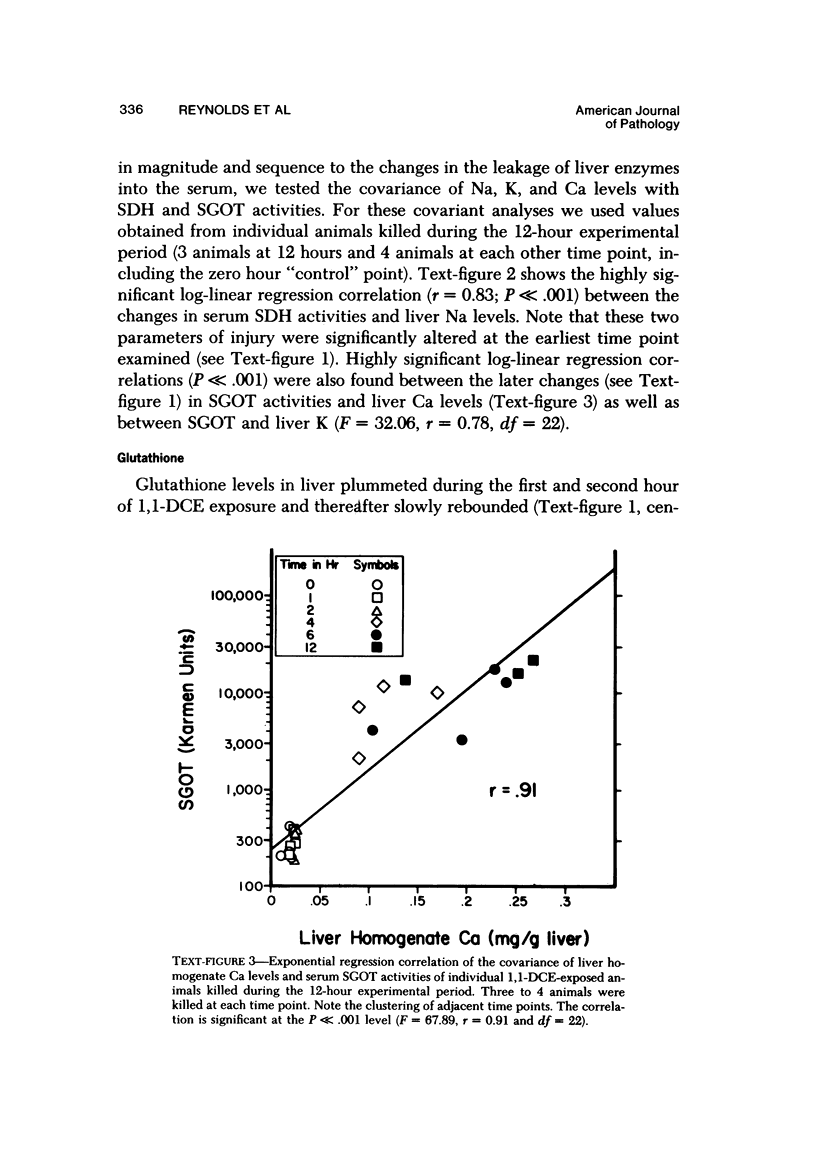
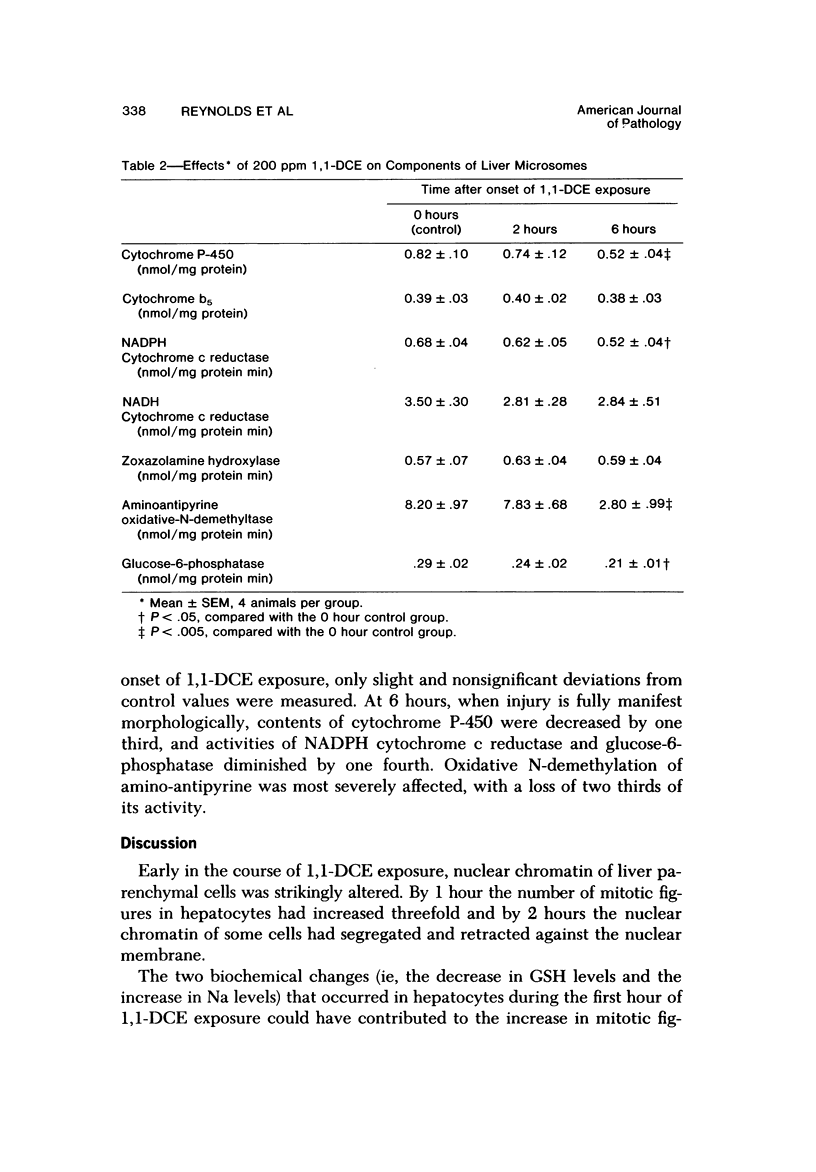
Exposure of fasted rats to 200 ppm 1,1-dichloroethylene (1,1-DCE) for 1-4 hours resulted in striking aberrations in hepatic Na, K, Ca, and GSH levels which preceded and/or accompanied catastrophic histologic alterations of the liver. Na levels began to rise during the first hour, and preceded the morphologically apparent injury. Ca levels increased markedly and K levels declined between the second and fourth hour of exposure, and accompanied the catastrophic morphologic alterations. GSH levels were rapidly depleted but began to recover before the end of the exposure to 1,1-DCE. Functions of components of the mixed-function oxidase system of the liver endoplasmic reticulum were not appreciably affected early in the course of 1,1-DCE exposure; but after injury became massive, cytochrome P-450 and oxidative N-demethylase were deactivated. Thus effects on the functional components of the endoplasmic reticulum mixed-function oxidase system do not appear to be primary events in 1,1-DCE cytotoxicity. In contrast, there were progressive declines in mitochondrial K and marked imbalances in mitochondrial Na, Zn, and Mg preceding the massive influx of Ca into the cell, indicating that mitochondria are involved early in he evolution of injurious molecular events elicited by this potent hepatotoxin.
Full text
PDF







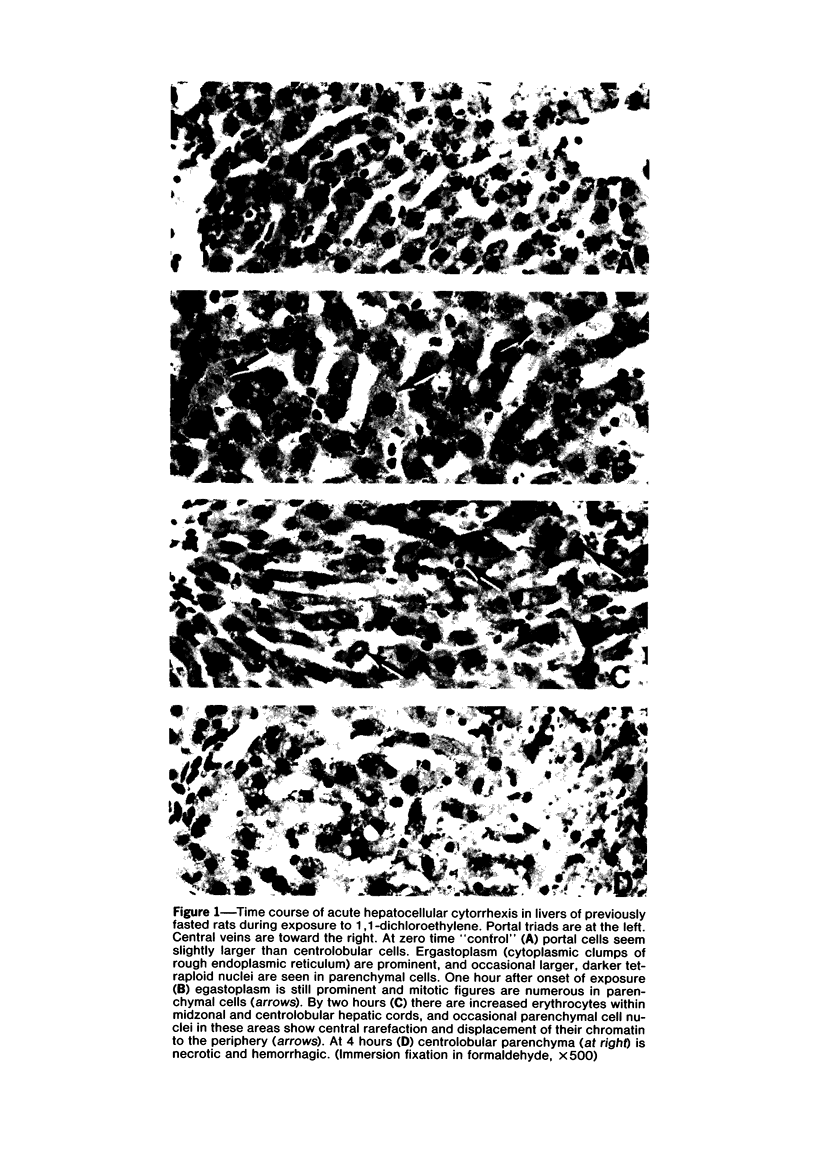
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Choie D. D., Richter G. W. G2 sub-population in mouse liver induced into mitosis by lead acetate. Cell Tissue Kinet. 1978 May;11(3):235–239. doi: 10.1111/j.1365-2184.1978.tb00891.x. [DOI] [PubMed] [Google Scholar]
- Harris E. J., Catlin G., Pressman B. C. Effect of transport-inducing antibiotics and other agents on potassium flux in mitochondria. Biochemistry. 1967 May;6(5):1360–1369. doi: 10.1021/bi00857a019. [DOI] [PubMed] [Google Scholar]
- Jaeger R. J., Conolly R. B., Murphy S. D. Diurnal variation of hepatic glutathione concentration and its correlation with 1,1-dichloroethylene inhalation toxicity in rats. Res Commun Chem Pathol Pharmacol. 1973 Sep;6(2):465–471. [PubMed] [Google Scholar]
- Jaeger R. J., Conolly R. B., Murphy S. D. Effect of 18 hr fast and glutathione depletion on 1,1-dichloroethylene-induced hepatotoxicity and lethality in rats. Exp Mol Pathol. 1974 Apr;20(2):187–198. doi: 10.1016/0014-4800(74)90053-7. [DOI] [PubMed] [Google Scholar]
- Jaeger R. J., Trabulus M. J., Murphy S. D. Biochemical effects of 1,1-dichloroethylene in rats: dissociation of its hepatotoxicity from a lipoperoxidative mechanism. Toxicol Appl Pharmacol. 1973 Mar;24(3):457–467. doi: 10.1016/0041-008x(73)90052-5. [DOI] [PubMed] [Google Scholar]
- Jenkins L. J., Jr, Trabulus M. J., Murphy S. D. Biochemical effects of 1,1-dichloroethylene in rats: comparison with carbon tetrachloride and 1,2-dichloroethylene. Toxicol Appl Pharmacol. 1972 Nov;23(3):501–510. doi: 10.1016/0041-008x(72)90052-x. [DOI] [PubMed] [Google Scholar]
- Jones B. K., Hathway D. E. The biological fate of vinylidene chloride in rats. Chem Biol Interact. 1978 Jan;20(1):27–41. doi: 10.1016/0009-2797(78)90078-9. [DOI] [PubMed] [Google Scholar]
- Judah J. D., Ahmed K., McLean A. E., Christie G. S. Ion transport in ethionine intoxication. Lab Invest. 1966 Jan;15(1 Pt 1):167–175. [PubMed] [Google Scholar]
- Koch K. S., Leffert H. L. Increased sodium ion influx is necessary to initiate rat hepatocyte proliferation. Cell. 1979 Sep;18(1):153–163. doi: 10.1016/0092-8674(79)90364-7. [DOI] [PubMed] [Google Scholar]
- LEACH L. J. A LABORATORY TEST CHAMBER FOR STUDYING AIR-BORNE MATERIALS. UR-629. UR Rep. 1963 Aug 2;86:1–12. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McKenna M. J., Watanabe P. G., Gehring P. J. Pharmacokinetics of vinylidene chloride in the rat. Environ Health Perspect. 1977 Dec;21:99–105. doi: 10.1289/ehp.772199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moslen M. T., Reynolds E. S., Szabo S. Enhancement of the metabolism and hepatotoxicity of trichloroethylene and perchloroethylene. Biochem Pharmacol. 1977 Mar 1;26(5):369–375. doi: 10.1016/0006-2952(77)90193-9. [DOI] [PubMed] [Google Scholar]
- Nath J., Rebhun J. I. Effects of caffeine and other methylxanthines on the development and metabolism of sea urchin eggs. Involvement of NADP and glutathione. J Cell Biol. 1976 Mar;68(3):440–450. doi: 10.1083/jcb.68.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki K., Shull K. H., Farber E. Effects of ethionine on adenosine triphosphate levels and ionic composition of liver cell nuclei. J Biol Chem. 1968 Sep 25;243(18):4661–4666. [PubMed] [Google Scholar]
- REYNOLDS E. S., THIERS R. E., VALLEE B. L. Mitochondrial function and metal content in carbon tetrachloride poisoning. J Biol Chem. 1962 Nov;237:3546–3551. [PubMed] [Google Scholar]
- Reynolds E. S., Moslen M. T. Damage to hepatic cellular membranes by chlorinated olefins with emphasis on synergism and antagonism. Environ Health Perspect. 1977 Dec;21:137–147. doi: 10.1289/ehp.7721137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds E. S., Moslen M. T., Szabo S., Jaeger R. J., Murphy S. D. Hepatotoxicity of vinyl chloride and 1,1-dichloroethylene. Role of mixed function oxidase system. Am J Pathol. 1975 Oct;81(1):219–236. [PMC free article] [PubMed] [Google Scholar]
- Reynolds E. S., Ree H. J., Moslen M. T. Liver parenchymal cell injury. IX. Phenobarbital potentiation of endoplasmic reticulum denaturation following carbon tetrachloride. Lab Invest. 1972 Mar;26(3):290–299. [PubMed] [Google Scholar]
- Thoma F., Koller T., Klug A. Involvement of histone H1 in the organization of the nucleosome and of the salt-dependent superstructures of chromatin. J Cell Biol. 1979 Nov;83(2 Pt 1):403–427. doi: 10.1083/jcb.83.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. M., Dattagupta N., Hogan M., Crothers D. M. Structural changes of nucleosomes in low-salt concentrations. Biochemistry. 1979 Sep 4;18(18):3960–3965. doi: 10.1021/bi00585a018. [DOI] [PubMed] [Google Scholar]



