Abstract
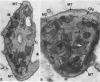
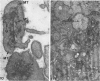
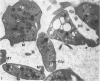
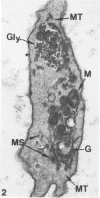
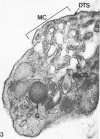
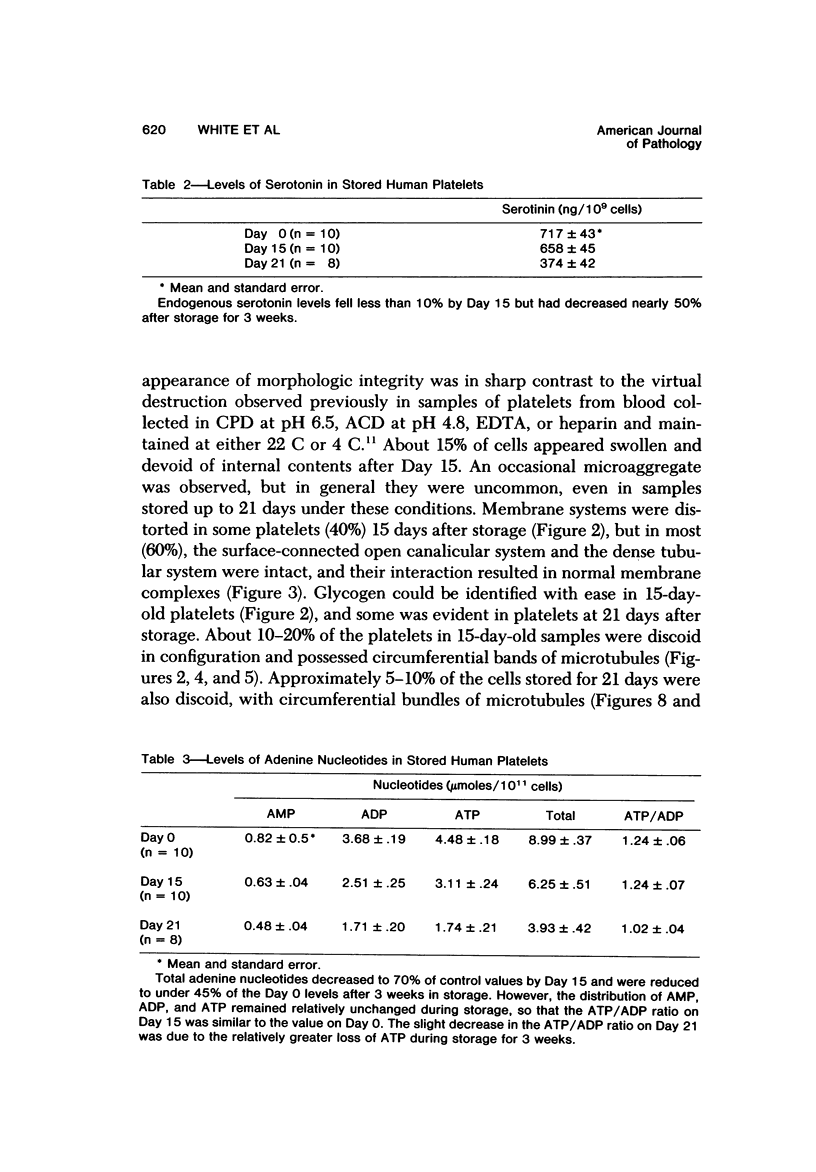
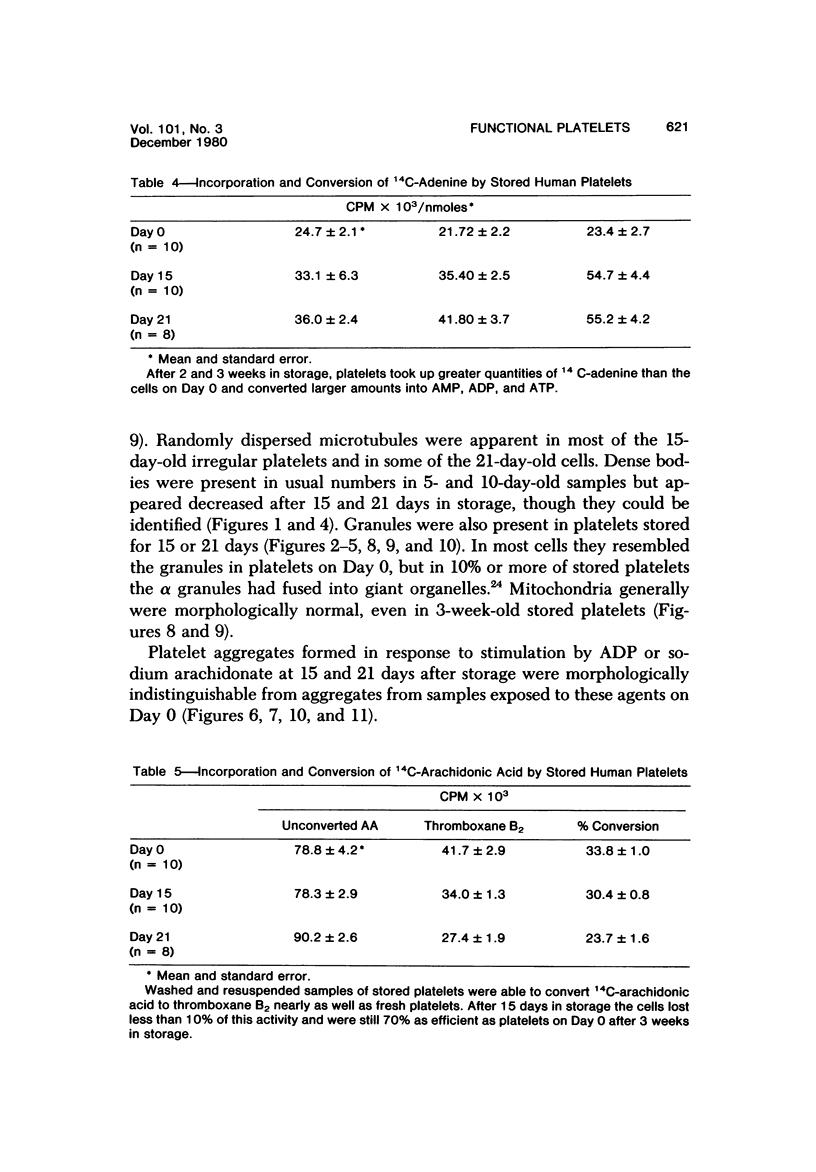
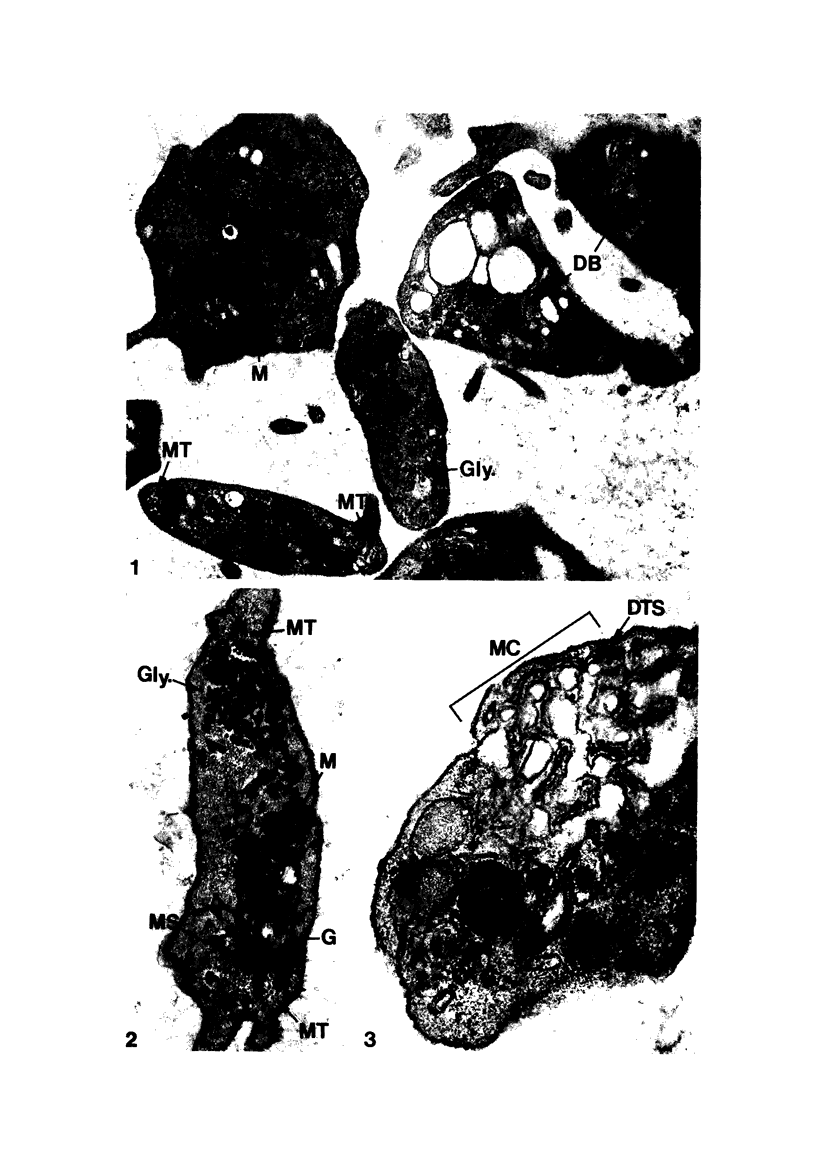
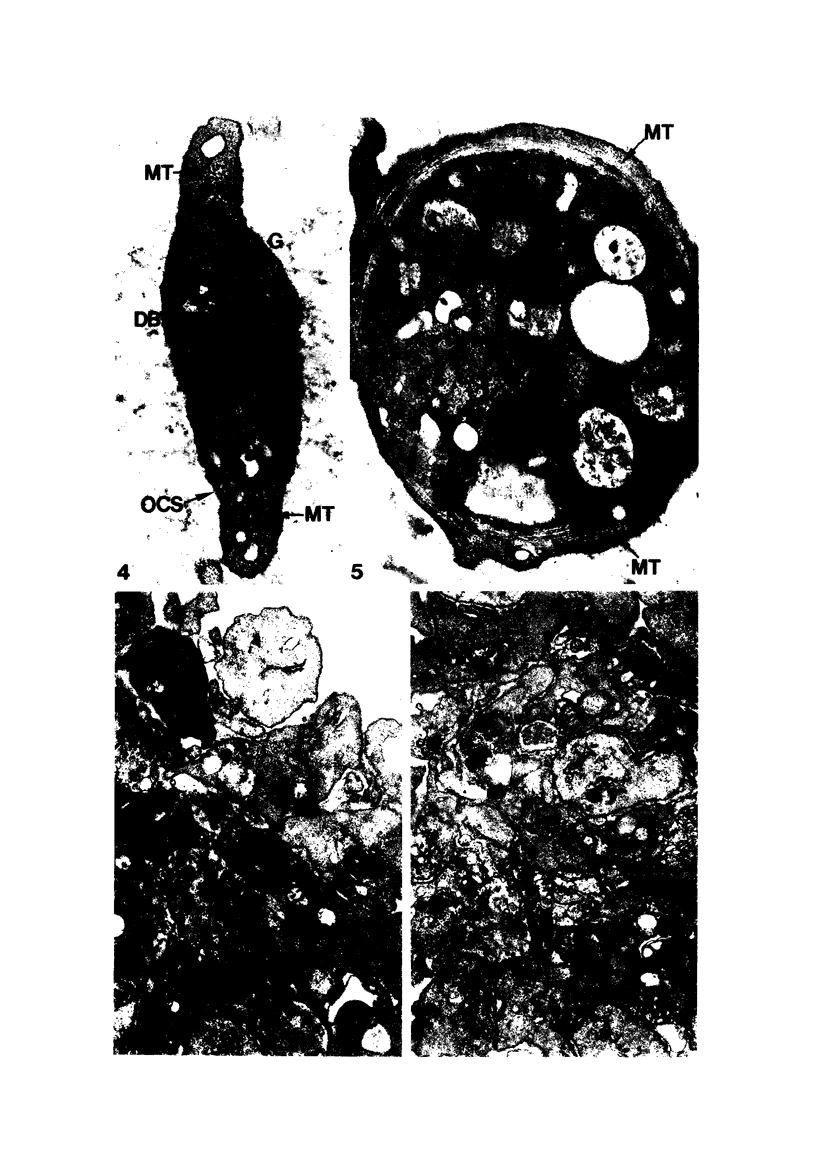
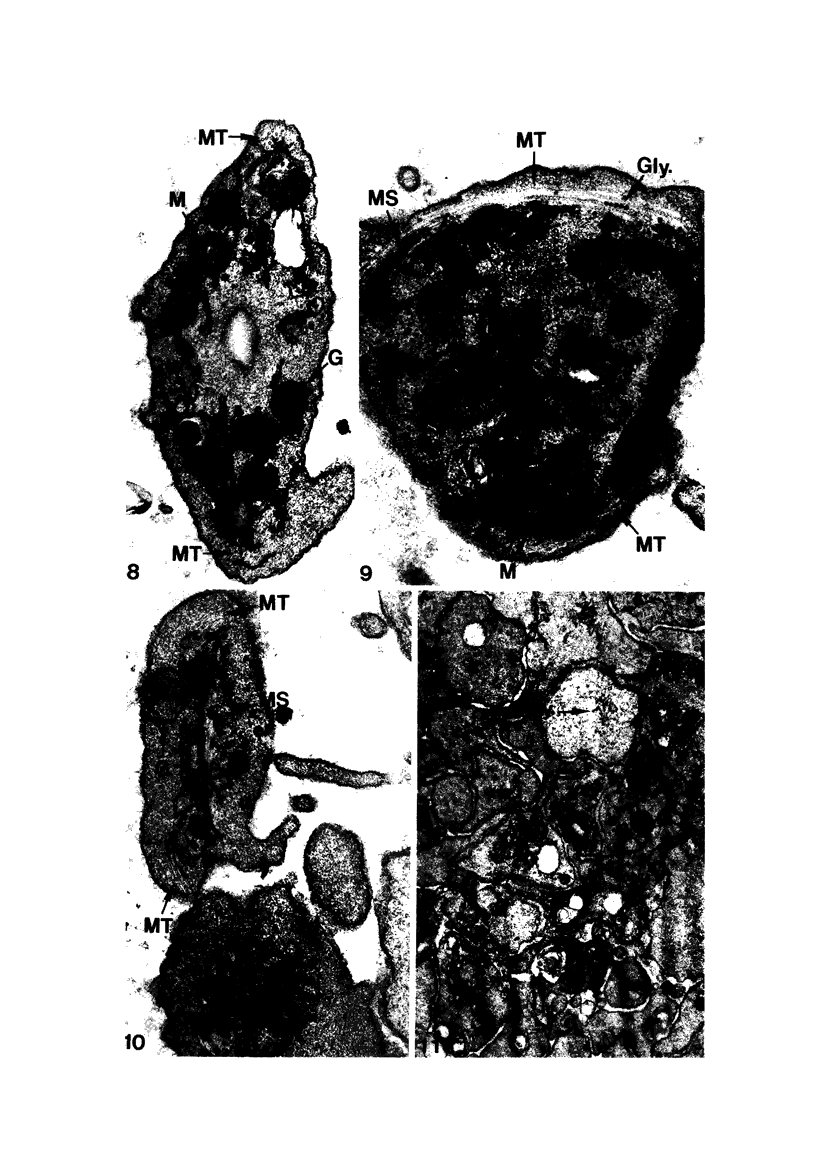
Blood platelets are notoriously difficult to preserve in vitro for long periods of time. Despite many efforts to solve the problem and improve conditions for storage, platelets lose their ability to respond to aggregating agents after 72--96 hours and are routinely discarded by blood banks after three days if not used for transfusion. The present study has evaluated the influence of raising the pH of the anticoagulant used to collect blood on the functional viability of platelets during storage at room temperature. Twenty-four samples of C-PRP were followed for 15 days and 12 samples for 21 days. Although platelet counts fell steadily during the 3-week storage period, a significant proportion of the cells remained viable. After 5--10 days the platelets responded as well to threshold concentrations of ADP and sodium arachidonate (SA) as on Day 0, and reactions to the same agents on Day 15 were nearly as impressive. Even on Day 21, responses to ADP and SA could still be elicited. Biochemical studies on samples stored for 15--21 days revealed normal levels of serotonin after 2 weeks and a fall of less than 30% after 3 weeks. The ability of the cells to convert 14C-arachidonic acid into thromboxane B2 was well maintained over the 3-week period. Adenine nucleotide levels fell 25% over 15 days and over 50% by 21 days, but the capacity of the cells to take up 14C-adenine and convert it to AMP, ADP, and ATP was increased, and ATP/ADP ratios were not greatly different from those on Day 0. Physical changes were apparent in most platelets by Day 15. However, 5--20% of platelets in 15--21-day-old samples were discoid in shape and contained circumferential bands of microtubules and small amounts of glycogen. The findings suggest that high pH during collection of blood, preparation of C-PRP, and early phases of storage may foster long-term preservation of viable platelets in vitro.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORN G. V. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962 Jun 9;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- Beutler E., West C. The storage of hard-packed red blood cells in citrate-phosphate-dextrose (CPD) and CPD-adenine (CPDA-1). Blood. 1979 Jul;54(1):280–284. [PubMed] [Google Scholar]
- Bressler N. M., Broekman M. J., Marcus A. J. Concurrent studies of oxygen consumption and aggregation in stimulated human platelets. Blood. 1979 Feb;53(2):167–178. [PubMed] [Google Scholar]
- Clawson C. C., White J. G. Platelet interaction with bacteria. I. Reaction phases and effects of inhibitors. Am J Pathol. 1971 Nov;65(2):367–380. [PMC free article] [PubMed] [Google Scholar]
- Frojmovic M. M. Quantitative parameterization of the light transmission properties of citrated, platelet-rich plasma as a function of platelet and adenosine diphosphate concentrations and temperature. J Lab Clin Med. 1973 Jul;82(1):137–153. [PubMed] [Google Scholar]
- Gerrard J. M., White J. G., Rao G. H., Townsend D. Localization of platelet prostaglandin production in the platelet dense tubular system. Am J Pathol. 1976 May;83(2):283–298. [PMC free article] [PubMed] [Google Scholar]
- Gorman R. R., Bunting S., Miller O. V. Modulation of human platelet adenylate cyclase by prostacyclin (PGX). Prostaglandins. 1977 Mar;13(3):377–388. doi: 10.1016/0090-6980(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handin R. I., Valeri C. R. Hemostatic effectiveness of platelets stored at 22 degrees C. N Engl J Med. 1971 Sep 2;285(10):538–543. doi: 10.1056/NEJM197109022851003. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Rozenberg M. C. Adenine nucleotide metabolism of blood platelets. 3. Adenine phosphoribosyl transferase and nucleotide formation from exogenous adenine. Biochim Biophys Acta. 1968 Apr 22;157(2):266–279. [PubMed] [Google Scholar]
- Kelton J. G., Blajchman M. A. Platelet transfusions. Can Med Assoc J. 1979 Nov 17;121(10):1353–1358. [PMC free article] [PubMed] [Google Scholar]
- Kunicki T. J., Tuccelli M., Becker G. A., Aster R. H. A study of variables affecting the quality of platelets stored at "room temperature". Transfusion. 1975 Sep-Oct;15(5):414–421. doi: 10.1046/j.1537-2995.1975.15576082215.x. [DOI] [PubMed] [Google Scholar]
- McGill M. Temperature cycling preserves platelet shape and enhances in vitro test scores during storage at 4 degrees. J Lab Clin Med. 1978 Dec;92(6):971–982. [PubMed] [Google Scholar]
- Moncada S., Higgs E. A., Vane J. R. Human arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregation. Lancet. 1977 Jan 1;1(8001):18–20. doi: 10.1016/s0140-6736(77)91655-5. [DOI] [PubMed] [Google Scholar]
- Murphy S., Gardner F. H. Effect of storage temperature on maintenance of platelet viability--deleterious effect of refrigerated storage. N Engl J Med. 1969 May 15;280(20):1094–1098. doi: 10.1056/NEJM196905152802004. [DOI] [PubMed] [Google Scholar]
- Rao G. H., Reddy K. R., Hagert K., White J. G. Influence of pH on the prostacyclin (PGI2) mediated inhibition of platelet function. Prostaglandins Med. 1980 Apr;4(4):263–273. doi: 10.1016/0161-4630(80)90021-x. [DOI] [PubMed] [Google Scholar]
- Rao G. H., White J. G., Jachimowicz A. A., Witkop C. J. An improved method for the extraction of endogenous platelet serotonin. J Lab Clin Med. 1976 Jan;87(1):129–137. [PubMed] [Google Scholar]
- Roth G. J., Stanford N., Majerus P. W. Acetylation of prostaglandin synthase by aspirin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3073–3076. doi: 10.1073/pnas.72.8.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slichter S. J., Harker L. A. Preparation and storage of platelet concentrates. Transfusion. 1976 Jan-Feb;16(1):8–12. doi: 10.1046/j.1537-2995.1976.16176130842.x. [DOI] [PubMed] [Google Scholar]
- Tateson J. E., Moncada S., Vane J. R. Effects of prostacyclin (PGX) on cyclic AMP concentrations in human platelets. Prostaglandins. 1977 Mar;13(3):389–397. doi: 10.1016/0090-6980(77)90019-3. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- Weiss H. J. Platelet physiology and abnormalities of platelet function (second of two parts). N Engl J Med. 1975 Sep 18;293(12):580–588. doi: 10.1056/NEJM197509182931204. [DOI] [PubMed] [Google Scholar]
- White J. G. Fine structural alterations induced in platelets by adenosine diphosphate. Blood. 1968 May;31(5):604–622. [PubMed] [Google Scholar]
- White J. G., Krivit W. An ultrastructural basis for the shape changes induced in platelets by chilling. Blood. 1967 Nov;30(5):625–635. [PubMed] [Google Scholar]
- White J. G. Ultrastructural studies of the gray platelet syndrome. Am J Pathol. 1979 May;95(2):445–462. [PMC free article] [PubMed] [Google Scholar]