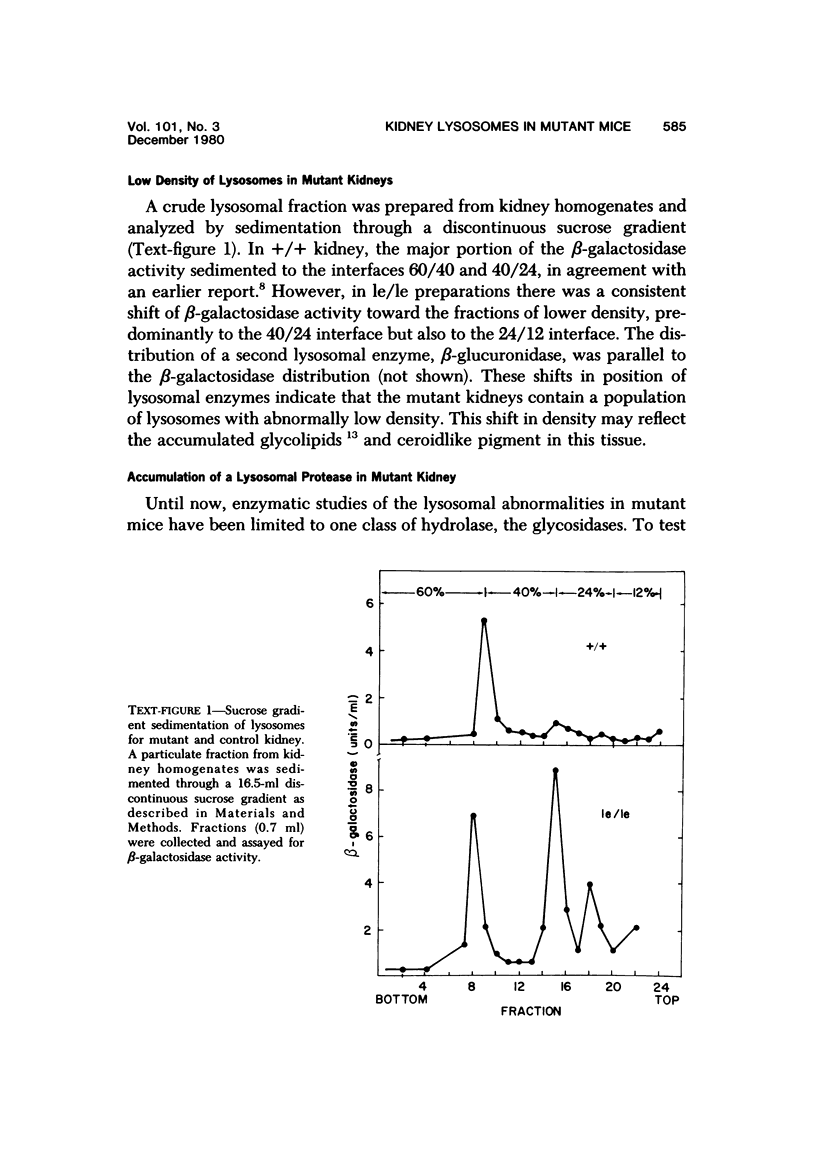
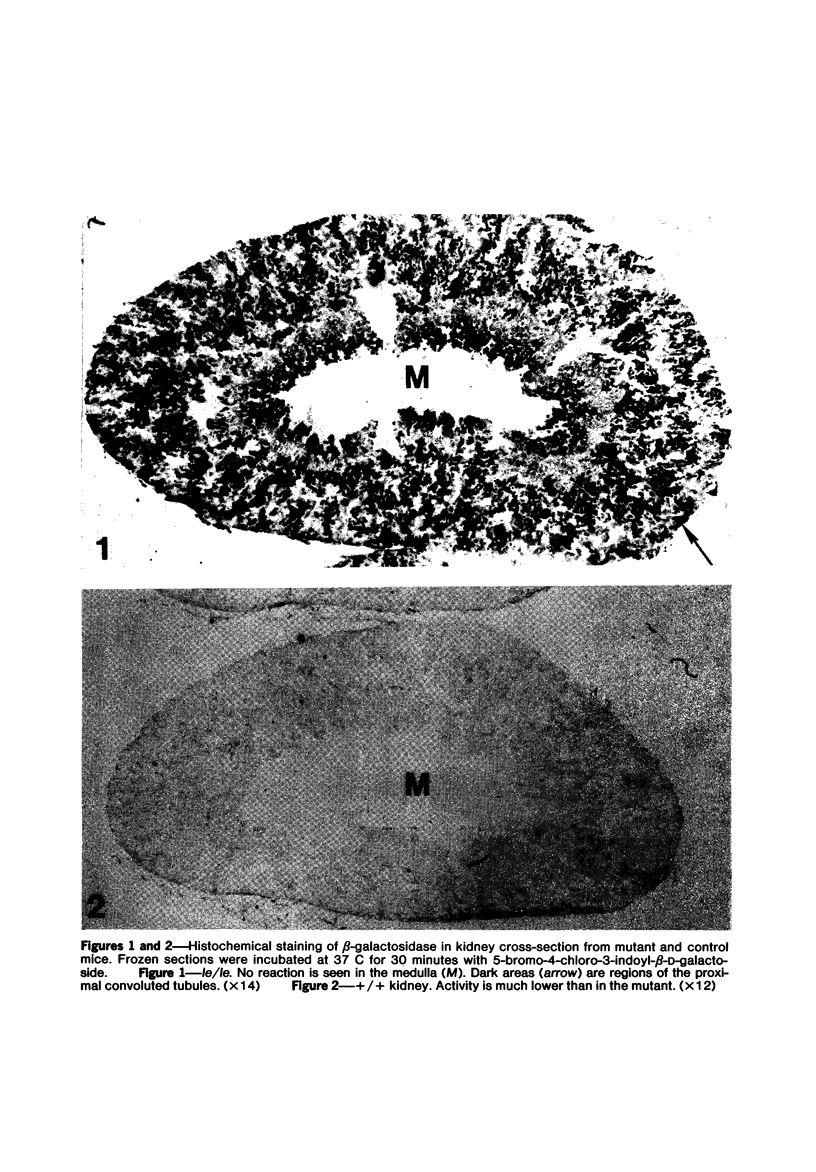
Abstract
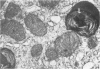
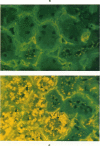
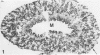
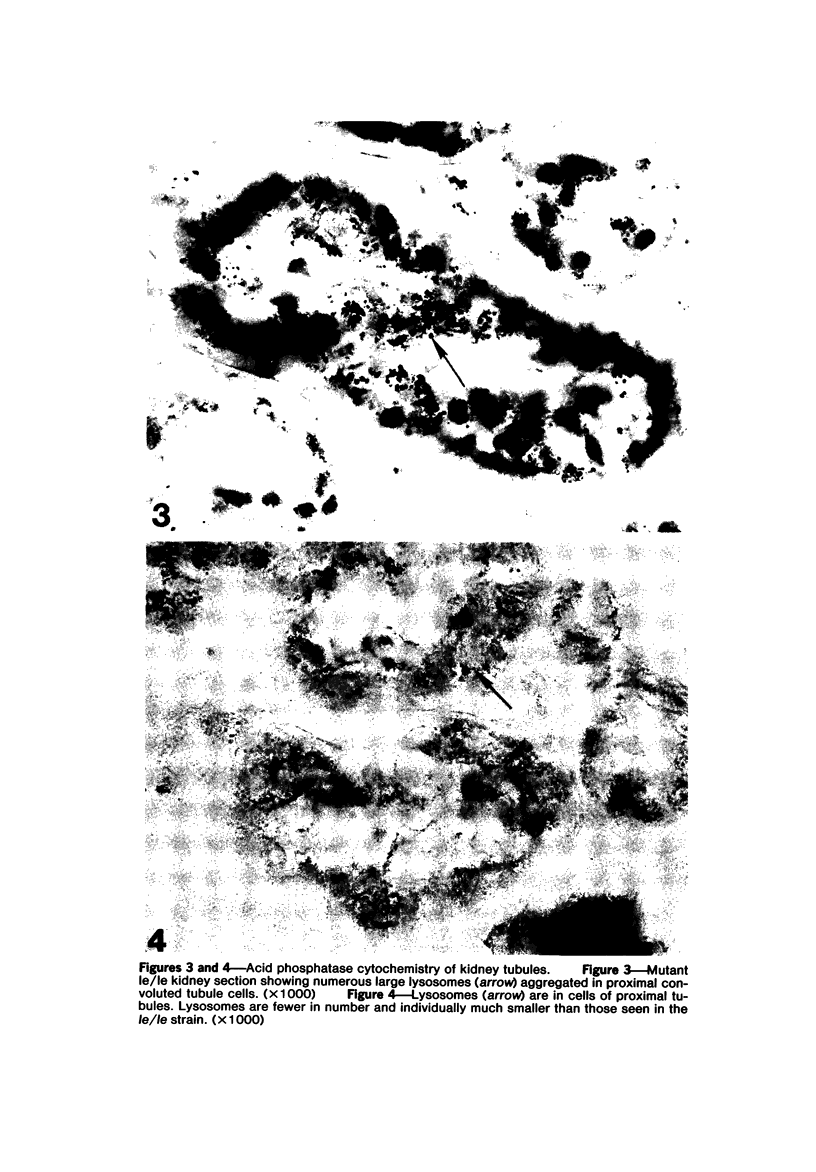
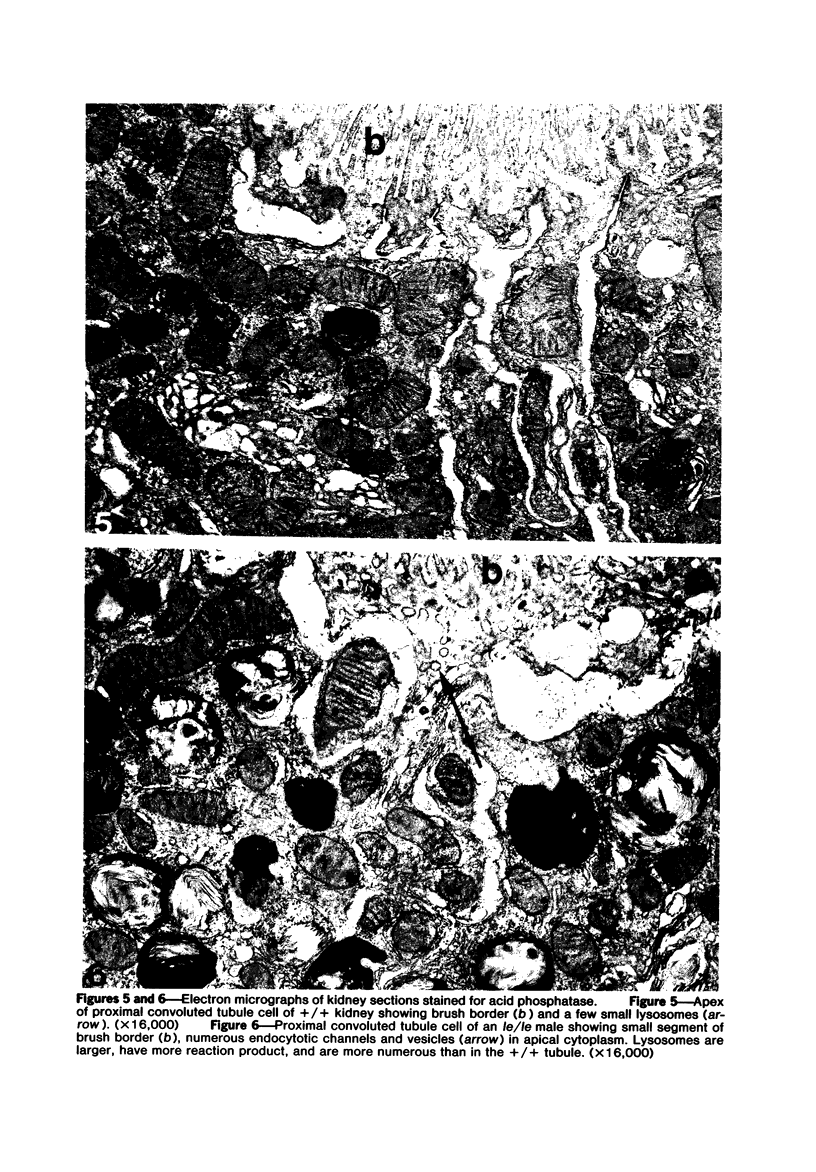
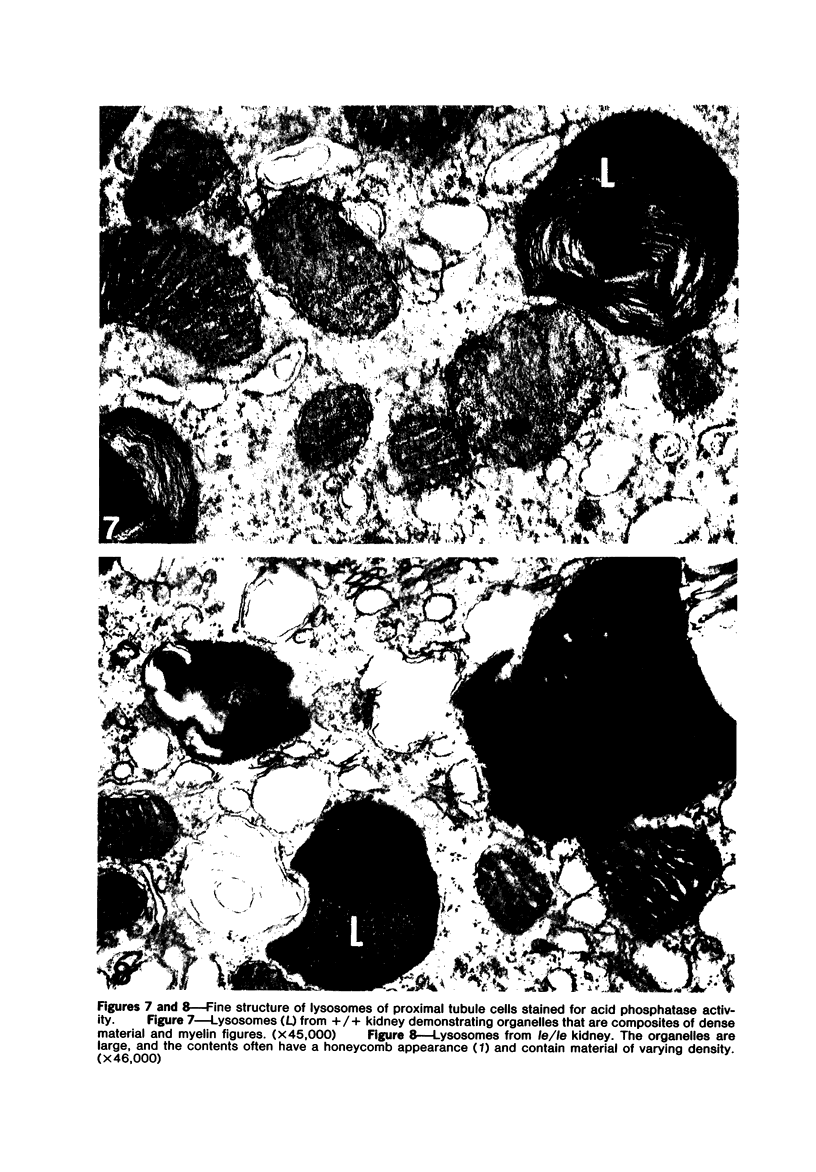
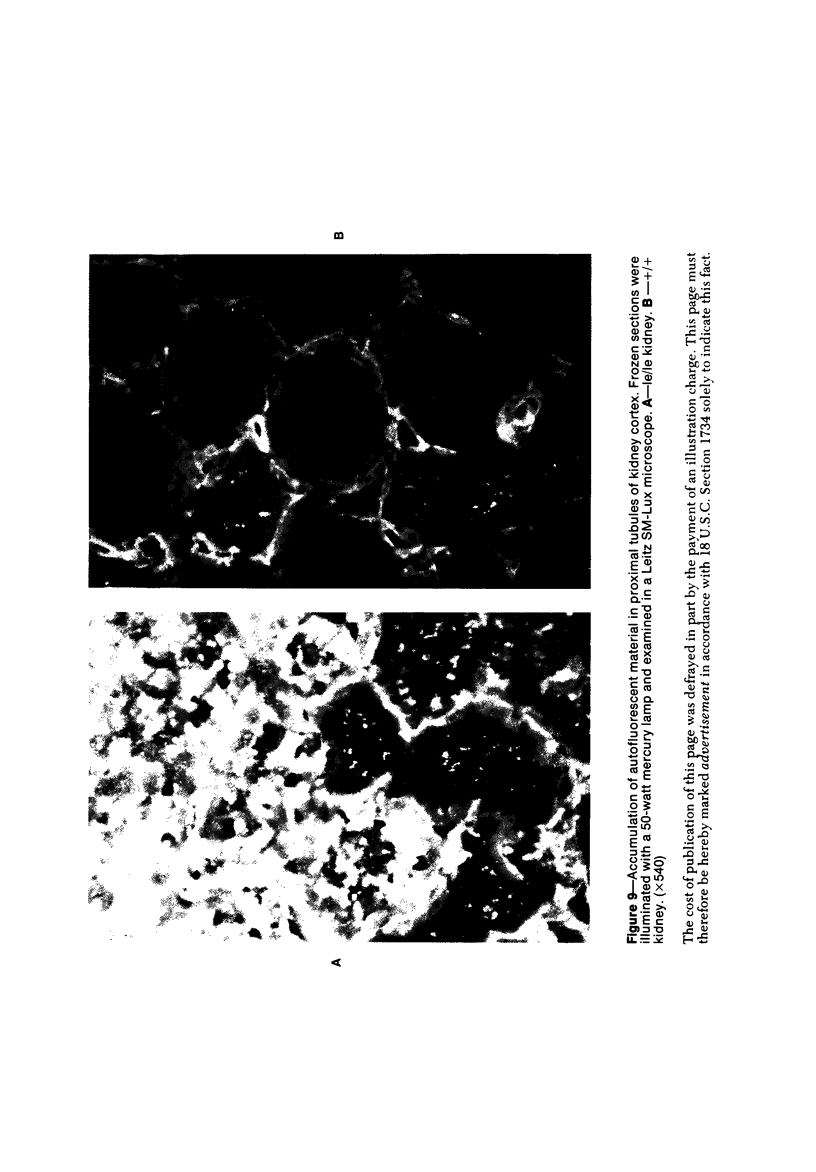
The light-ear mutation in the mouse may serve as a useful model for the human inherited oculocutaneous albinisms such as the Hermansky-Pudlak and Chédiak-Higashi syndromes. The authors have investigated the kidney lysosomes of le/le mutant mice by histochemical methods. A striking increase in the staining reaction for the lysosomal enzymes beta-galactosidase and acid-phosphatase was evident in kidney cortex of the mutant mice, in comparison with +/+ controls. The lysosomal protease, cathepsin C, is also found to be elevated in the mutant. By light microscopy, there appeared to be an increase in the number of lysosomes in mutant kidney. Electron microscopy revealed the presence of large, multilamellar granules in proximal tubule cells. Analysis of sedimentation through sucrose gradients demonstrated the presence of a low-density population of lysosomes in the mutant kidney. In addition, a striking accumulation of ceroidlike pigment was observed. The molecular lesions responsible for the melanolysosomal syndromes in mice and man are still unidentified.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Felton J., Meisler M., Paigen K. A locus determining beta-galactosidase activity in the mouse. J Biol Chem. 1974 May 25;249(10):3267–3272. [PubMed] [Google Scholar]
- Koenig H., Goldstone A., Hughes C. Lysosomal enzymuria in the testosterone-treated mouse. A manifestation of cell defecation of residual bodies. Lab Invest. 1978 Oct;39(4):329–341. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaVail M. M., Sidman R. L. C57BL-6J mice with inherited retinal degeneration. Arch Ophthalmol. 1974 May;91(5):394–400. doi: 10.1001/archopht.1974.03900060406015. [DOI] [PubMed] [Google Scholar]
- Lusis A. J., Breen G. A., Paigen K. Nongenetic heterogeneity of mouse beta-galactosidase. J Biol Chem. 1977 Jul 10;252(13):4613–4618. [PubMed] [Google Scholar]
- McDonald J. K., Ellis S., Reilly T. J. Properties of dipeptidyl arylamidase I of the pituitary. Chloride and sulfhydryl activation of seryltyrosyl-beta-naphthylamide hydrolysis. J Biol Chem. 1966 Apr 10;241(7):1494–1501. [PubMed] [Google Scholar]
- Meisler M. H. Synthesis and secretion of kidney beta-galactosidase in mutant le/le mice. J Biol Chem. 1978 May 10;253(9):3129–3134. [PubMed] [Google Scholar]
- Meisler M., Paigen K. Coordinated development of -glucuronidase and -galactosidase in mouse organs. Science. 1972 Sep 8;177(4052):894–896. doi: 10.1126/science.177.4052.894. [DOI] [PubMed] [Google Scholar]
- Novak E. K., Swank R. T. Lysosomal dysfunctions associated with mutations at mouse pigment genes. Genetics. 1979 May;92(1):189–204. doi: 10.1093/genetics/92.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver C., Essner E., Zimring A., Haimes H. Age-related accumulation of ceroid-like pigment in mice with Chediak-Higashi syndrome. Am J Pathol. 1976 Aug;84(2):225–238. [PMC free article] [PubMed] [Google Scholar]
- Peters B. P., Goldstein I. J. The use of fluorescein-conjugated Bandeiraea simplicifolia B4-isolectin as a histochemical reagent for the detection of alpha-D-galactopyranosyl groups. Their occurrence in basement membranes. Exp Cell Res. 1979 May;120(2):321–334. doi: 10.1016/0014-4827(79)90392-6. [DOI] [PubMed] [Google Scholar]
- Piccini A. E., Jahreis G. P., Novak E. K., Swank R. T. Intracellular distribution of lysosomal enzymes in the mouse pigment mutants pale ear and pallid. Mol Cell Biochem. 1980 Jun 18;31(2):89–95. doi: 10.1007/BF00240814. [DOI] [PubMed] [Google Scholar]