Abstract
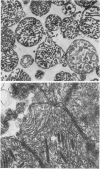
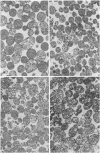
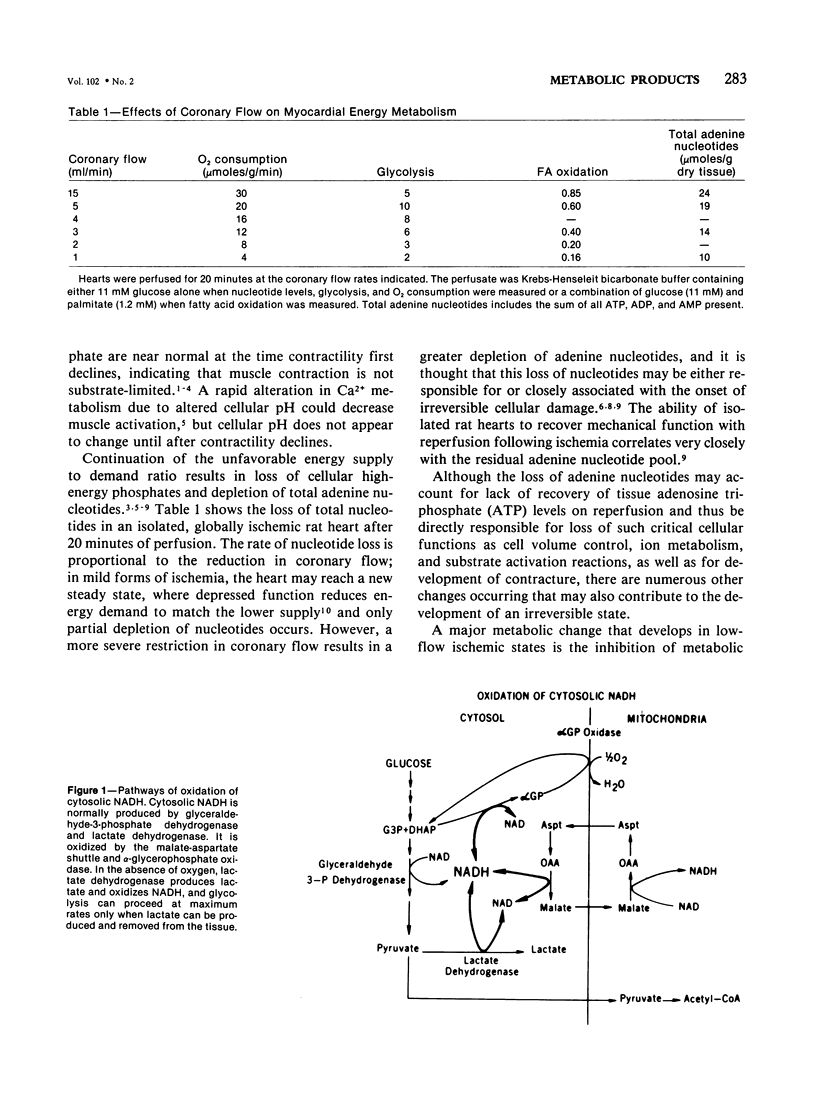
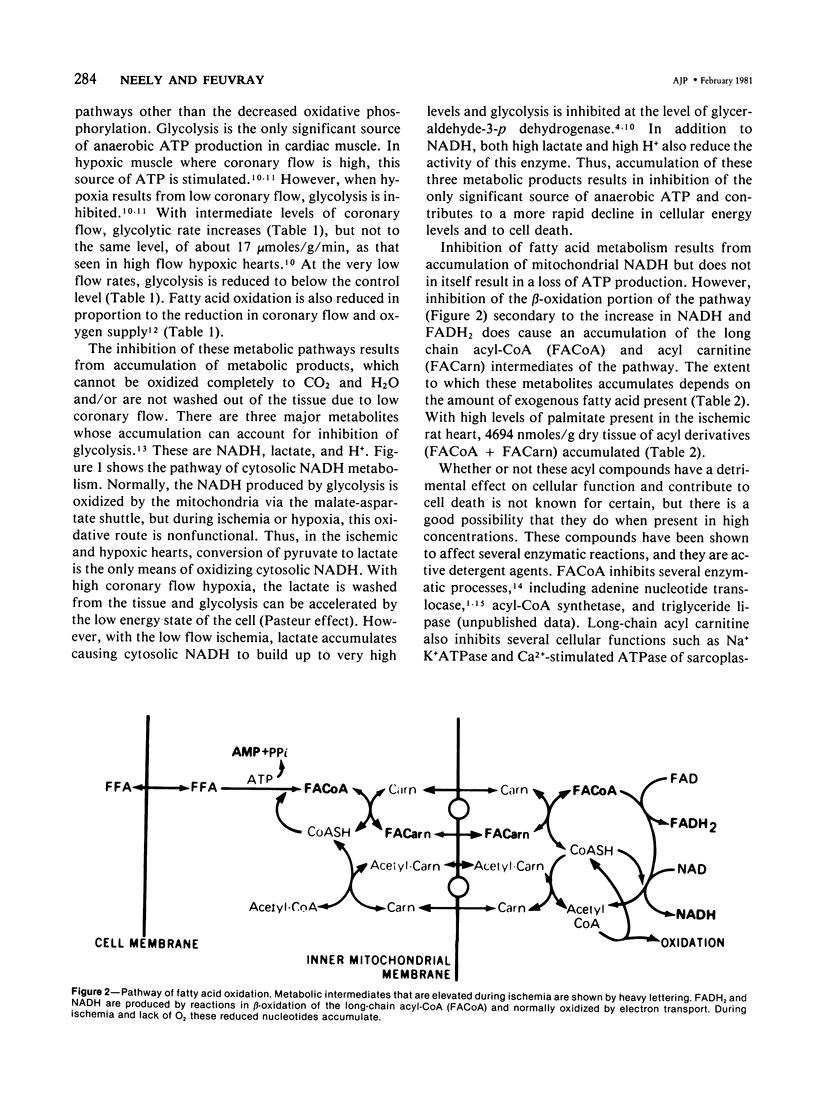
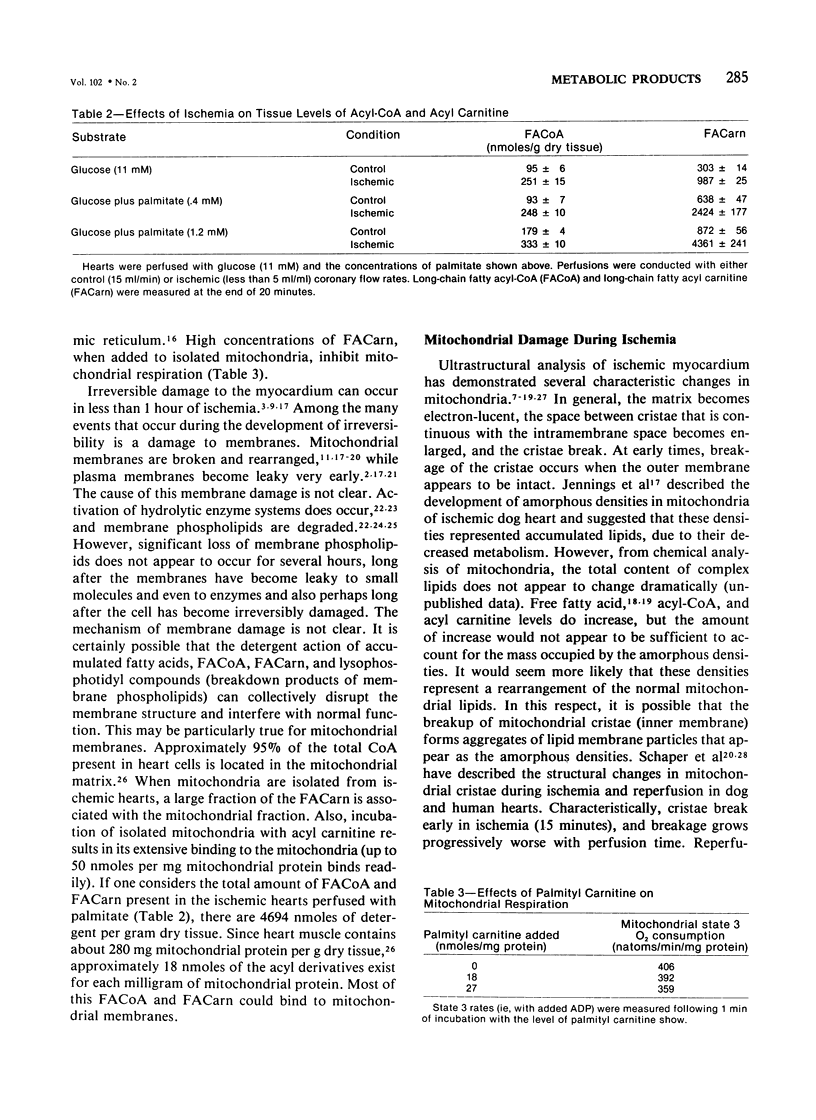
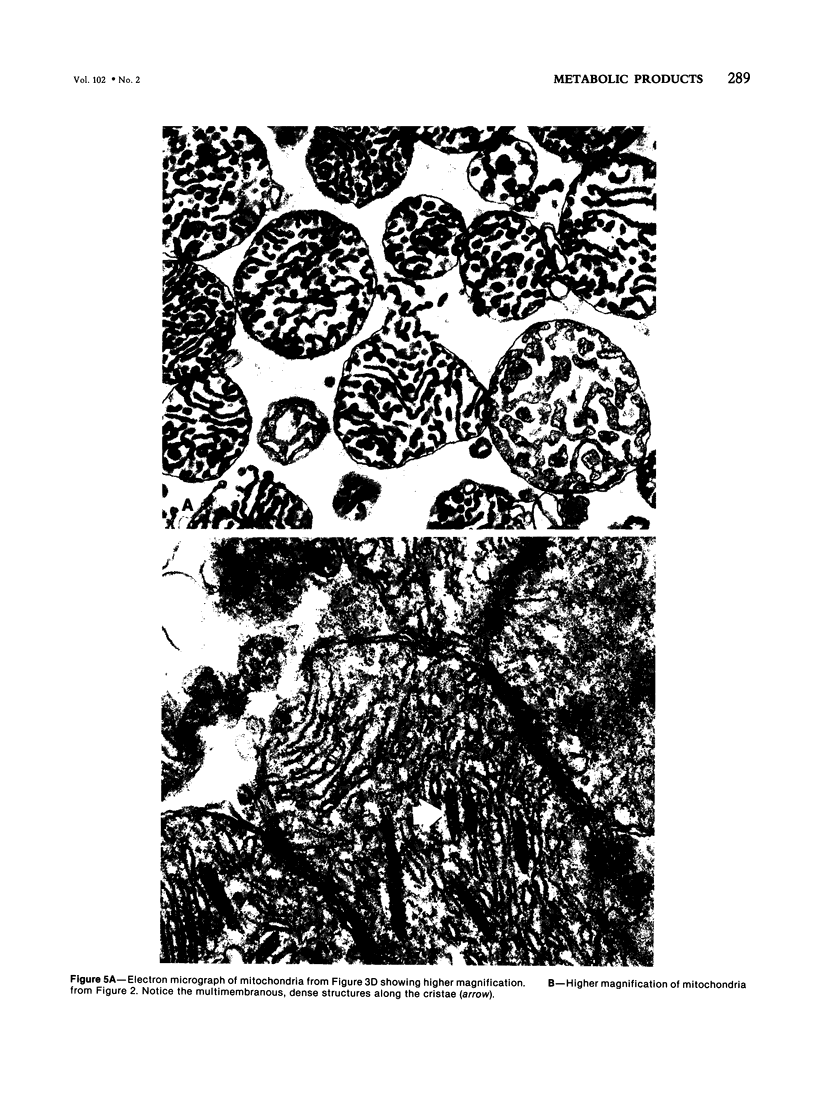
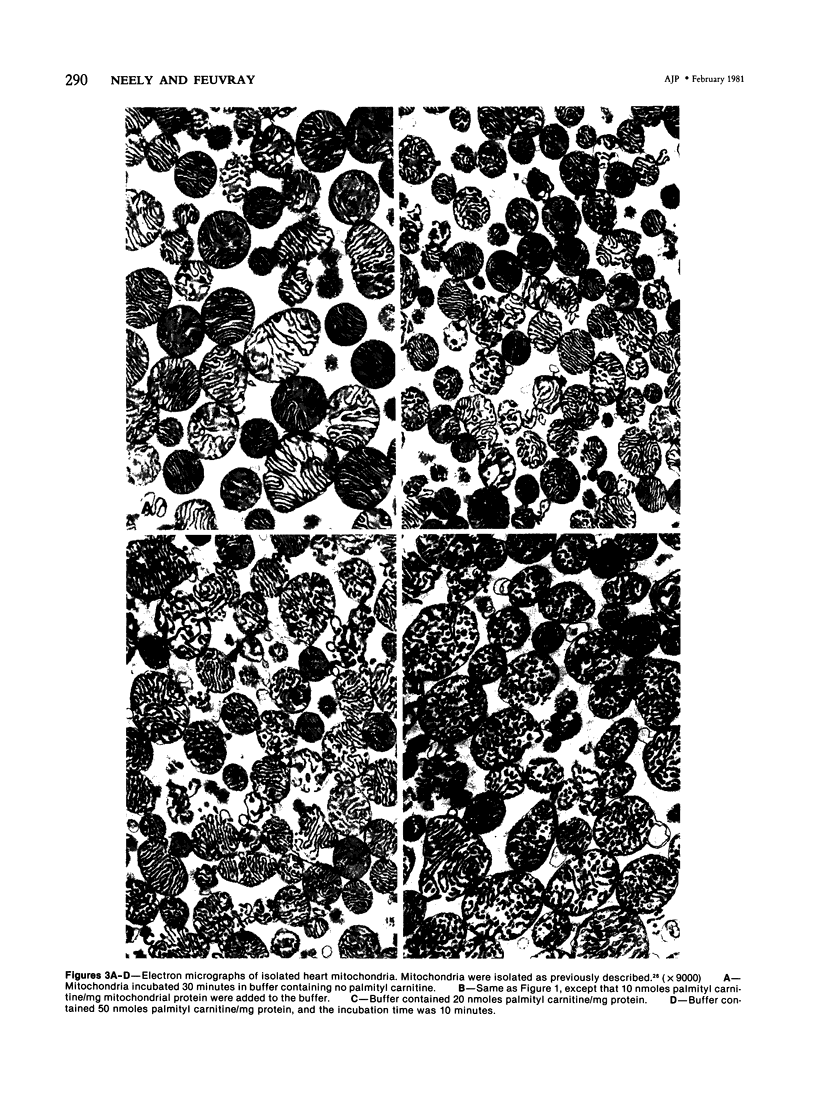
Metabolic products accumulate in ischemic myocardium secondary to reduced coronary flow, which prevents adequate washout of vascular spaces, and to reduced oxidative metabolism. The most notable products that accumulate are NADH, H+, lactate, CO2, long-chain acyl-CoA, and long-chain acyl carnitine. These products interfere with the production of ATP and the functioning of the myocardium. Glycolytic production of ATP is inhibited by accumulation of NADH, H+, and lactate. Mitochondrial and plasma membrane function may be altered by the acyl esters of CoA and carnitine. Mitochondrial membranes become structurally distorted and fragmented, and lipid-containing amorphous densities appear in the matrix. Structural alterations of mitochondria occur more frequently in hearts receiving high concentrations of fatty acids and correlate with high tissue levels of acyl esters of CoA and carnitine. Addition of acyl carnitine to mitochondria isolated from normal hearts results in nodulose-appearing cristae and fragmentation of mitochondrial membranes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashraf M. Ultrastructural alterations in the mitochondrial membranes of ischemic myocardium as revealed by freeze-fracture technique. J Mol Cell Cardiol. 1978 Jun;10(6):535–543. doi: 10.1016/0022-2828(78)90012-3. [DOI] [PubMed] [Google Scholar]
- Bricknell O. L., Opie L. H. Effects of substrates on tissue metabolic changes in the isolated rat heart during underperfusion and on release of lactate dehydrogenase and arrhythmias during reperfusion. Circ Res. 1978 Jul;43(1):102–115. doi: 10.1161/01.res.43.1.102. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Cain M. E., Witkowski F. X., Price D. A., Sobel B. E. Potential arrhythmogenic electrophysiological derangements in canine Purkinje fibers induced by lysophosphoglycerides. Circ Res. 1979 Jun;44(6):822–832. doi: 10.1161/01.res.44.6.822. [DOI] [PubMed] [Google Scholar]
- Gudbjarnason S., Mathes P., Ravens K. G. Functional compartmentation of ATP and creatine phosphate in heart muscle. J Mol Cell Cardiol. 1970 Sep;1(3):325–339. doi: 10.1016/0022-2828(70)90009-x. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Humphrey S. M., Bullock G. R. The oxygen paradox and the calcium paradox: two facets of the same problem? J Mol Cell Cardiol. 1978 Jul;10(7):641–668. doi: 10.1016/s0022-2828(78)80004-2. [DOI] [PubMed] [Google Scholar]
- Idell-Wenger J. A., Grotyohann L. W., Neely J. R. Coenzyme A and carnitine distribution in normal and ischemic hearts. J Biol Chem. 1978 Jun 25;253(12):4310–4318. [PubMed] [Google Scholar]
- Jennings R. B. Early phase of myocardial ischemic injury and infarction. Am J Cardiol. 1969 Dec;24(6):753–765. doi: 10.1016/0002-9149(69)90464-0. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Hawkins H. K., Lowe J. E., Hill M. L., Klotman S., Reimer K. A. Relation between high energy phosphate and lethal injury in myocardial ischemia in the dog. Am J Pathol. 1978 Jul;92(1):187–214. [PMC free article] [PubMed] [Google Scholar]
- Kao R., Rannels D. E., Morgan H. E. Effects of anoxia and severe ischemia on the turnover of myocardial proteins. Acta Med Scand Suppl. 1976;587:117–123. doi: 10.1111/j.0954-6820.1976.tb05873.x. [DOI] [PubMed] [Google Scholar]
- Kübler W., Spieckermann P. G. Changes in glycolysis and in high-energy phosphates during myocardial ischemia with intermittent coronary perfusion. Cardiology. 1971;56(1):100–107. doi: 10.1159/000169349. [DOI] [PubMed] [Google Scholar]
- Lochner A., Kotzé J. C., Benade A. J., Gevers W. Mitochondrial oxidative phosphorylation in low-flow hypoxia: role of free fatty acids. J Mol Cell Cardiol. 1978 Sep;10(9):857–875. doi: 10.1016/0022-2828(78)90394-2. [DOI] [PubMed] [Google Scholar]
- Lochner A., Kotzé J. C., Gevers W. Mitochondrial oxidative phosphorylation in myocardial anoxia: effects of albumin. J Mol Cell Cardiol. 1976 Jun;8(6):465–480. doi: 10.1016/0022-2828(76)90020-1. [DOI] [PubMed] [Google Scholar]
- Mochizuki S., Neely J. R. Control of glyceraldehyde-3-phosphate dehydrogenase in cardiac muscle. J Mol Cell Cardiol. 1979 Mar;11(3):221–236. doi: 10.1016/0022-2828(79)90437-1. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Liebermeister H., Morgan H. E. Effect of pressure development on membrane transport of glucose in isolated rat heart. Am J Physiol. 1967 Apr;212(4):815–822. doi: 10.1152/ajplegacy.1967.212.4.815. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Rovetto M. J., Whitmer J. T., Morgan H. E. Effects of ischemia on function and metabolism of the isolated working rat heart. Am J Physiol. 1973 Sep;225(3):651–658. doi: 10.1152/ajplegacy.1973.225.3.651. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Whitmer J. T., Rovetto M. J. Effect of coronary blood flow on glycolytic flux and intracellular pH in isolated rat hearts. Circ Res. 1975 Dec;37(6):733–741. doi: 10.1161/01.res.37.6.733. [DOI] [PubMed] [Google Scholar]
- Opie L. H. Role of carnitine in fatty acid metabolism of normal and ischemic myocardium. Am Heart J. 1979 Mar;97(3):375–388. doi: 10.1016/0002-8703(79)90440-x. [DOI] [PubMed] [Google Scholar]
- Pearce F. J., Forster J., DeLeeuw G., Williamson J. R., Tutwiler G. F. Inhibition of fatty acid oxidation and in normal and hypoxic perfused rat hearts by 2-tetradecylglycidic acid. J Mol Cell Cardiol. 1979 Sep;11(9):893–915. doi: 10.1016/0022-2828(79)90483-8. [DOI] [PubMed] [Google Scholar]
- Pitts B. J., Tate C. A., Van Winkle W. B., Wood J. M., Entman M. L. Palmitylcarnitine inhibition of the calcium pump in cardiac sarcoplasmic reticulum: a possible role in myocardial ischemia. Life Sci. 1978 Jul 24;23(4):391–401. doi: 10.1016/0024-3205(78)90025-5. [DOI] [PubMed] [Google Scholar]
- Reibel D. K., Rovetto M. J. Myocardial ATP synthesis and mechanical function following oxygen deficiency. Am J Physiol. 1978 May;234(5):H620–H624. doi: 10.1152/ajpheart.1978.234.5.H620. [DOI] [PubMed] [Google Scholar]
- Rovetto M. J., Whitmer J. T., Neely J. R. Comparison of the effects of anoxia and whole heart ischemia on carbohydrate utilization in isolated working rat hearts. Circ Res. 1973 Jun;32(6):699–711. doi: 10.1161/01.res.32.6.699. [DOI] [PubMed] [Google Scholar]
- Schaper J., Hehrlein F., Schlepper M., Thiedemann K. U. Ultrastructural alterations during ischemia and reperfusion in human hearts during cardiac surgery. J Mol Cell Cardiol. 1977 Mar;9(3):175–189. doi: 10.1016/0022-2828(77)90028-1. [DOI] [PubMed] [Google Scholar]
- Schaper J., Mulch J., Winkler B., Schaper W. Ultrastructural, functional, and biochemical criteria for estimation of reversibility of ischemic injury: a study on the effects of global ischemia on the isolated dog heart. J Mol Cell Cardiol. 1979 Jun;11(6):521–541. doi: 10.1016/0022-2828(79)90428-0. [DOI] [PubMed] [Google Scholar]
- Sobel B. E., Corr P. B., Robison A. K., Goldstein R. A., Witkowski F. X., Klein M. S. Accumulation of lysophosphoglycerides with arrhythmogenic properties in ischemic myocardium. J Clin Invest. 1978 Sep;62(3):546–553. doi: 10.1172/JCI109159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen C., Deleeuw G., Rich T., Williamson J. R. Effects of acidosis and ischemia on contractility and intracellular pH of rat heart. Circ Res. 1977 Dec;41(6):849–858. doi: 10.1161/01.res.41.6.849. [DOI] [PubMed] [Google Scholar]
- Steenbergen C., Deleeuw G., Williamson J. R. Analysis of control of glycolysis in ischemic hearts having heterogeneous zones of anoxia. J Mol Cell Cardiol. 1978 Jul;10(7):617–639. doi: 10.1016/s0022-2828(78)80003-0. [DOI] [PubMed] [Google Scholar]
- Weglicki W. B., Owens K., Urschel C. W., Serur J. R., Sonnenblick E. H. Hydrolysis of myocardial lipids during acidosis and ischemia. Recent Adv Stud Cardiac Struct Metab. 1973;3:781–793. [PubMed] [Google Scholar]
- Whitmer J. T., Idell-Wenger J. A., Rovetto M. J., Neely J. R. Control of fatty acid metabolism in ischemic and hypoxic hearts. J Biol Chem. 1978 Jun 25;253(12):4305–4309. [PubMed] [Google Scholar]
- Wildenthal K. Lysosomal alterations in ischemic myocardium: result or cause of myocellular damage? J Mol Cell Cardiol. 1978 Jul;10(7):595–603. doi: 10.1016/s0022-2828(78)80001-7. [DOI] [PubMed] [Google Scholar]
- de Leiris J., Feuvray D. Ischaemia-induced damage in the working rat heart preparation: the effect of perfusate substrate composition upon subendocardial ultrastructure of the ischaemic left ventricular wall. J Mol Cell Cardiol. 1977 May;9(5):365–373. doi: 10.1016/s0022-2828(77)80003-5. [DOI] [PubMed] [Google Scholar]