Abstract
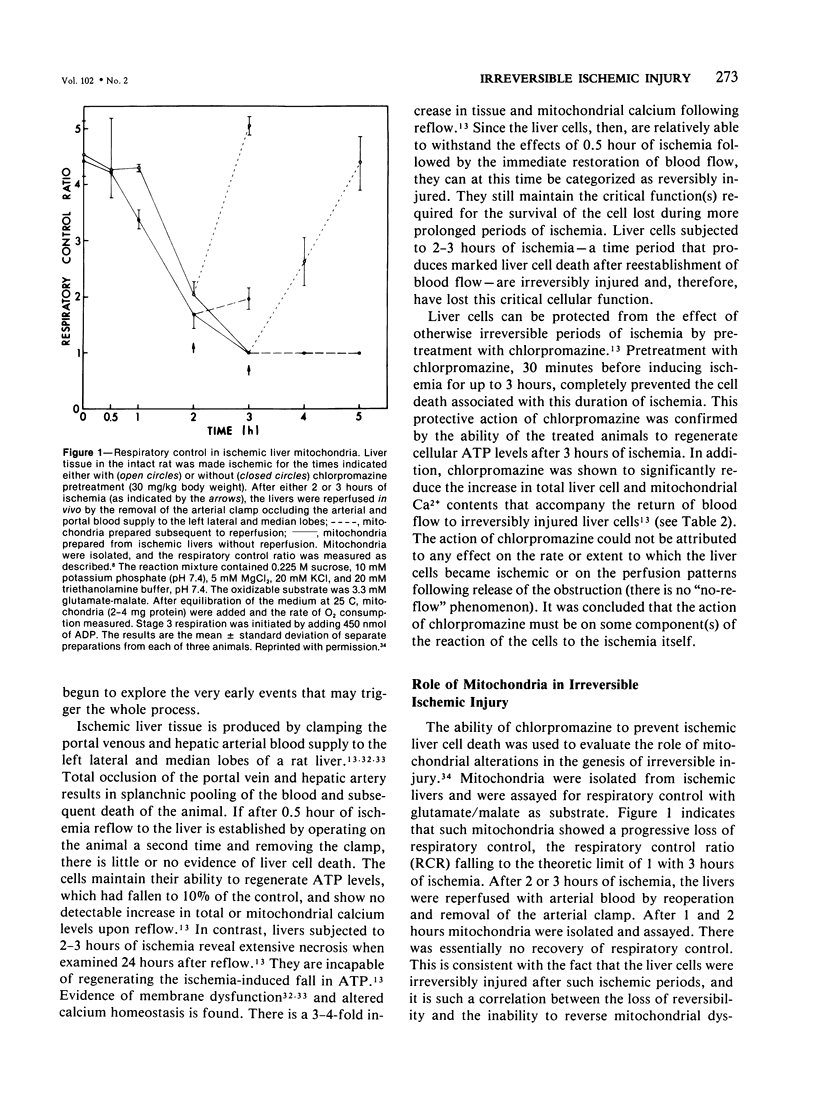
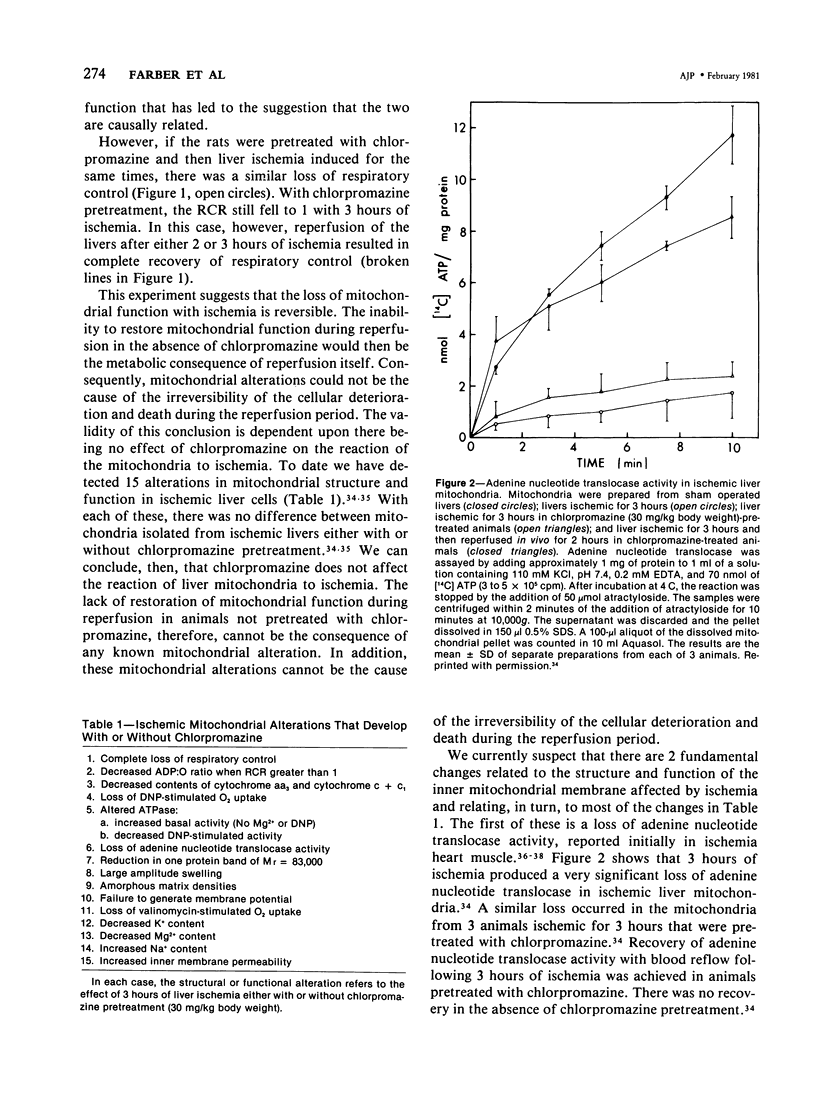
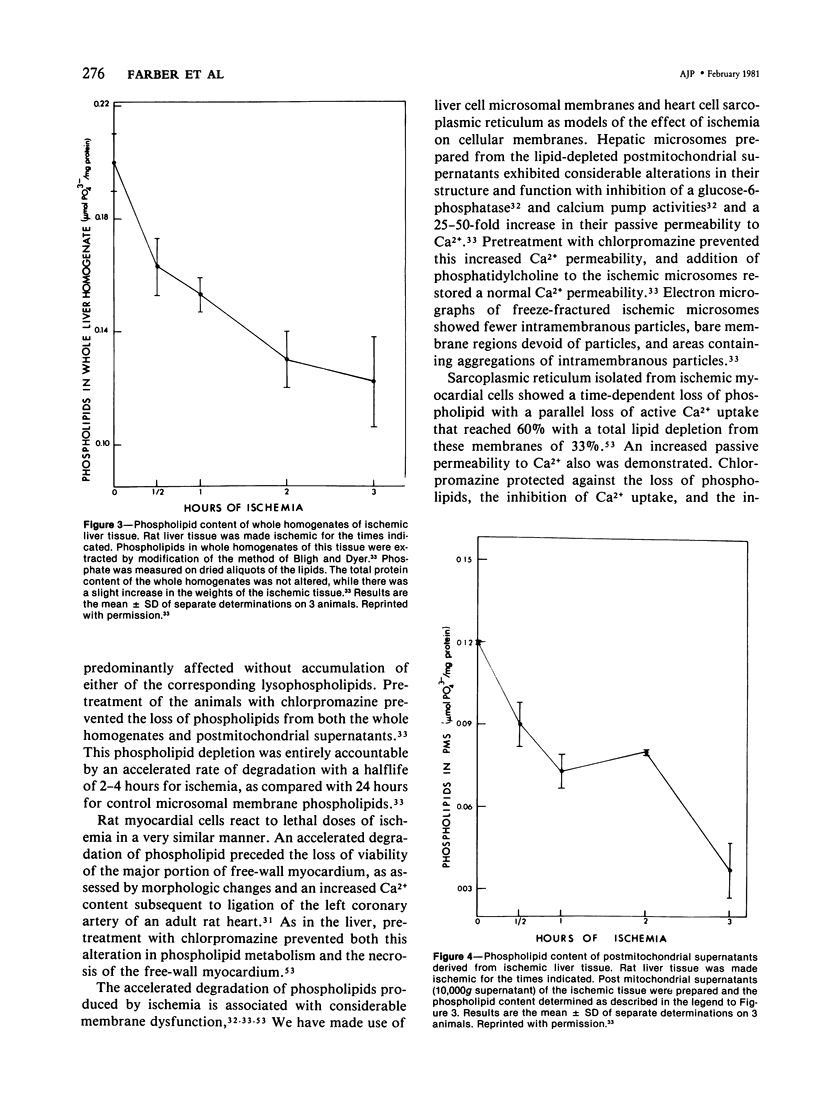
Cells made ischemic rapidly manifest many distinct structural and functional alterations as a consequence of the depletion of their energy stores. In attempting to determine which of these are causally related to the eventual cell death, the authors have emphasized the relationship to the reversibility of the ischemic injury. Two phenomena have consistently characterized irreversibly in contrast to reversibly injured ischemic cells: the inability to restore mitochondrial function and evidence of plasma membrane damage. Studies in the authors' laboratory are reviewed that have focused on the pathogenesis, biochemical nature, and the relationship to irreversible cell injury of both of these alterations. A number of mitochondrial abnormalities are related to changes in long-chain acyl-CoA metabolism with inhibition of adenine nucleotide translocation and potentiation of a Ca2+-dependent increase in the permeability of the inner mitochondrial membrane. These changes are reversible upon reoxygenation only when the large increase in intracellular Ca2+ content that accompanies the phospholipid depletion from other cellular membranes is prevented. This disorder in phospholipid metabolism is felt to be the critical lesion that produces irreversible cell injury in ischemia. It affects the endoplasmic and sarcoplasmic reticular membranes of liver and myocardial cells, respectively, and probably the plasma membranes of both. It is prevented by pretreatment with chlorpromazine. An activation of endogenous phospholipases by an elevated, cytosolic free Ca2+ ion concentration is suggested as the mechanism underlying this phospholipid disturbance. The central role of intracellular Ca2+ in the initiation and functional consequences of ischemic cell injury are emphasized.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., MALLUCCI L. HISTOCHEMICAL STUDIES OF LYSOSOMES AND LYSOSOMAL ENZYMES IN VIRUS-INFECTED CELL CULTURES. J Exp Med. 1965 Mar 1;121:463–476. doi: 10.1084/jem.121.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLISON A. C., YOUNG M. R. UPTAKE OF DYES AND DRUGS BY LIVING CELLS IN CULTURE. Life Sci. 1964 Dec;3:1407–1414. doi: 10.1016/0024-3205(64)90082-7. [DOI] [PubMed] [Google Scholar]
- Adams R. J., Cohen D. W., Gupte S., Johnson J. D., Wallick E. T., Wang T., Schwartz A. In vitro effects of palmitylcarnitine on cardiac plasma membrane Na,K-ATPase, and sarcoplasmic reticulum Ca2+-ATPase and Ca2+ transport. J Biol Chem. 1979 Dec 25;254(24):12404–12410. [PubMed] [Google Scholar]
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A., 3rd, Wright R. L., Kowada M., Thurston J. M., Majno G. Cerebral ischemia. II. The no-reflow phenomenon. Am J Pathol. 1968 Feb;52(2):437–453. [PMC free article] [PubMed] [Google Scholar]
- Ashraf M., Halverson C. A. Structural changes in the freeze-fractured sarcolemma of ischemic myocardium. Am J Pathol. 1977 Sep;88(3):583–594. [PMC free article] [PubMed] [Google Scholar]
- Beatrice M. C., Palmer J. W., Pfeiffer D. R. The relationship between mitochondrial membrane permeability, membrane potential, and the retention of Ca2+ by mitochondria. J Biol Chem. 1980 Sep 25;255(18):8663–8671. [PubMed] [Google Scholar]
- Beller G. A., Conroy J., Smith T. W. Ischemia-induced alterations in myocardial (Na+ + K+)-ATPase and cardiac glycoside binding. J Clin Invest. 1976 Feb;57(2):341–350. doi: 10.1172/JCI108285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blok M. C., van der Neut-Kok E. C., van Deenen L. L., de Gier J. The effect of chain length and lipid phase transitions on the selective permeability properties of liposomes. Biochim Biophys Acta. 1975 Oct 6;406(2):187–196. doi: 10.1016/0005-2736(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Burton K. P., Hagler H. K., Templeton G. H., Willerson J. T., Buja L. M. Lanthanum probe studies of cellular pathophysiology induced by hypoxia in isolated cardiac muscle. J Clin Invest. 1977 Dec;60(6):1289–1302. doi: 10.1172/JCI108888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien K. R., Abrams J., Pfau R. G., Farber J. L. Prevention by chlorpromazine of ischemic liver cell death. Am J Pathol. 1977 Sep;88(3):539–557. [PMC free article] [PubMed] [Google Scholar]
- Chien K. R., Abrams J., Serroni A., Martin J. T., Farber J. L. Accelerated phospholipid degradation and associated membrane dysfunction in irreversible, ischemic liver cell injury. J Biol Chem. 1978 Jul 10;253(13):4809–4817. [PubMed] [Google Scholar]
- Chien K. R., Farber J. L. Microsomal membrane dysfunction in ischemic rat liver cells. Arch Biochem Biophys. 1977 Apr 15;180(1):191–198. doi: 10.1016/0003-9861(77)90025-x. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Peau R. G., Farber J. L. Ischemic myocardial cell injury. Prevention by chlorpromazine of an accelerated phospholipid degradation and associated membrane dysfunction. Am J Pathol. 1979 Dec;97(3):505–529. [PMC free article] [PubMed] [Google Scholar]
- Chien K. R., Sherman S. C., Mittnacht S., Jr, Farber J. L. Microsomal membrane structure and function subsequent to calcium activation of an endogenous phospholipase. Arch Biochem Biophys. 1980 Dec;205(2):614–622. doi: 10.1016/0003-9861(80)90145-9. [DOI] [PubMed] [Google Scholar]
- Coleman S. E., Duggan J., Hackett R. L. Plasma membrane changes in freeze-fractured rat kidney cortex following renal ischemia. Lab Invest. 1976 Jul;35(1):63–70. [PubMed] [Google Scholar]
- Decker R. S., Poole A. R., Crie J. S., Dingle J. T., Wildenthal K. Lysosomal alterations in hypoxic and reoxygenated hearts. II. Immunohistochemical and biochemical changes in cathepsin D. Am J Pathol. 1980 Feb;98(2):445–456. [PMC free article] [PubMed] [Google Scholar]
- Decker R. S., Poole A. R., Griffin E. E., Dingle J. T., Wildenthal K. Altered distribution of lysosomal cathepsin D in ischemic myocardium. J Clin Invest. 1977 May;59(5):911–921. doi: 10.1172/JCI108713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker R. S., Wildenthal K. Lysosomal alterations in hypoxic and reoxygenated hearts. I. Ultrastructural and cytochemical changes. Am J Pathol. 1980 Feb;98(2):425–444. [PMC free article] [PubMed] [Google Scholar]
- Decker R. S., Wildenthal K. Sequential lysosomal alterations during cardiac ischemia. II. Ultrastructural and cytochemical changes. Lab Invest. 1978 Jun;38(6):662–673. [PubMed] [Google Scholar]
- Duppel W., Dahl G. Effect of phase transition on the distribution of membrane-associated particles in microsomes. Biochim Biophys Acta. 1976 Mar 19;426(3):408–417. doi: 10.1016/0005-2736(76)90386-2. [DOI] [PubMed] [Google Scholar]
- Farber J. L., Martin J. T., Chien K. R. Irreversible ischemic cell injury. Prevention by chlorpromazine of the aggregation of the intramembranous particles of rat liver plasma membranes. Am J Pathol. 1978 Sep;92(3):713–732. [PMC free article] [PubMed] [Google Scholar]
- Flores J., DiBona D. R., Beck C. H., Leaf A. The role of cell swelling in ischemic renal damage and the protective effect of hypertonic solute. J Clin Invest. 1972 Jan;51(1):118–126. doi: 10.1172/JCI106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., DiBona D. R., Frega N., Leaf A. Cell volume regulation and ischemic tissue damage. J Membr Biol. 1972 Dec 29;10(3):331–343. doi: 10.1007/BF01867864. [DOI] [PubMed] [Google Scholar]
- Fukuzawa K., Ikeno H., Tokumura A., Tsukatani H. Effect of alpha-tocopherol incorporation of glucose permeability and phase transition of lecithin liposomes. Chem Phys Lipids. 1979 Jan;23(1):13–21. doi: 10.1016/0009-3084(79)90019-7. [DOI] [PubMed] [Google Scholar]
- GREENAWALT J. W., ROSSI C. S., LEHNINGER A. L. EFFECT OF ACTIVE ACCUMULATION OF CALCIUM AND PHOSPHATE IONS ON THE STRUCTURE OF RAT LIVER MITOCHONDRIA. J Cell Biol. 1964 Oct;23:21–38. doi: 10.1083/jcb.23.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaja G., Ferrero M. E., Piccoletti R., Bernelli-Zazzera A. Phosphorylation and redox states in ischemic liver. Exp Mol Pathol. 1973 Oct;19(2):248–265. doi: 10.1016/0014-4800(73)90083-x. [DOI] [PubMed] [Google Scholar]
- Harris R. A., Farmer B., Ozawa T. Inhibition of the mitochondrial adenine nucleotide transport system by oleyl CoA. Arch Biochem Biophys. 1972 May;150(1):199–209. doi: 10.1016/0003-9861(72)90027-6. [DOI] [PubMed] [Google Scholar]
- Haworth R. A., Hunter D. R. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys. 1979 Jul;195(2):460–467. doi: 10.1016/0003-9861(79)90372-2. [DOI] [PubMed] [Google Scholar]
- Hoffstein S., Gennaro D. E., Weissmann G., Hirsch J., Streuli F., Fox A. C. Cytochemical localization of lysosomal enzyme activity in normal and ischemic dog myocardium. Am J Pathol. 1975 May;79(2):193–206. [PMC free article] [PubMed] [Google Scholar]
- Hunter D. R., Haworth R. A., Southard J. H. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem. 1976 Aug 25;251(16):5069–5077. [PubMed] [Google Scholar]
- Hunter D. R., Haworth R. A. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch Biochem Biophys. 1979 Jul;195(2):453–459. doi: 10.1016/0003-9861(79)90371-0. [DOI] [PubMed] [Google Scholar]
- Hunter D. R., Haworth R. A. The Ca2+-induced membrane transition in mitochondria. III. Transitional Ca2+ release. Arch Biochem Biophys. 1979 Jul;195(2):468–477. doi: 10.1016/0003-9861(79)90373-4. [DOI] [PubMed] [Google Scholar]
- Höchli M., Hackenbrock C. R. Thermotropic lateral translational motion of intramembrane particles in the inner mitochondrial membrane and its inhibition by artificial peripheral proteins. J Cell Biol. 1977 Feb;72(2):278–291. doi: 10.1083/jcb.72.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idell-Wenger J. A., Grotyohann L. W., Neely J. R. Coenzyme A and carnitine distribution in normal and ischemic hearts. J Biol Chem. 1978 Jun 25;253(12):4310–4318. [PubMed] [Google Scholar]
- Ingwall J. S., DeLuca M., Sybers H. D., Wildenthal K. Fetal mouse hearts: a model for studying ischemia. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2809–2813. doi: 10.1073/pnas.72.7.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K. Permeability properties of liposomes prepared from dipalmitoyllecithin, dimyristoyllecithin, egg lecithin, rat liver lecithin and beef brain sphingomyelin. Biochim Biophys Acta. 1974 Mar 29;339(3):390–402. doi: 10.1016/0005-2736(74)90166-7. [DOI] [PubMed] [Google Scholar]
- JENNINGS R. B., SOMMERS H. M., KALTENBACH J. P., WEST J. J. ELECTROLYTE ALTERATIONS IN ACUTE MYOCARDIAL ISCHEMIC INJURY. Circ Res. 1964 Mar;14:260–269. doi: 10.1161/01.res.14.3.260. [DOI] [PubMed] [Google Scholar]
- James R., Branton D. Lipid- and temperature-dependent structural changes in Acholeplasma laidlawii cell membranes. Biochim Biophys Acta. 1973 Oct 25;323(3):378–390. doi: 10.1016/0005-2736(73)90183-1. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Ganote C. E., Kloner R. A., Whalen D. A., Jr, Hamilton D. G. Explosive swelling of myocardial cells irreversibly injured by transient ischemia. Recent Adv Stud Cardiac Struct Metab. 1975;6:405–413. [PubMed] [Google Scholar]
- Jennings R. B., Ganote C. E., Reimer K. A. Ischemic tissue injury. Am J Pathol. 1975 Oct;81(1):179–198. [PMC free article] [PubMed] [Google Scholar]
- Judah J. D., Ahmed K., McLean A. E., Christie G. S. Ion transport in ethionine intoxication. Lab Invest. 1966 Jan;15(1 Pt 1):167–175. [PubMed] [Google Scholar]
- Kane A. B., Stanton R. P., Raymond E. G., Dobson M. E., Knafelc M. E., Farber J. L. Dissociation of intracellular lysosomal rupture from the cell death caused by silica. J Cell Biol. 1980 Dec;87(3 Pt 1):643–651. doi: 10.1083/jcb.87.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane A. B., Young E. E., Schanne F. A., Farber J. L. Calcium dependence of phalloidin-induced liver cell death. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1177–1180. doi: 10.1073/pnas.77.2.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleemann W., McConnell H. M. Lateral phase separations in Escherichia coli membranes. Biochim Biophys Acta. 1974 Apr 29;345(2):220–230. doi: 10.1016/0005-2736(74)90260-0. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Ganote C. E., Whalen D. A., Jr, Jennings R. B. Effect of a transient period of ischemia on myocardial cells. II. Fine structure during the first few minutes of reflow. Am J Pathol. 1974 Mar;74(3):399–422. [PMC free article] [PubMed] [Google Scholar]
- Laiho K. U., Trump B. F. The relationship between cell viability and changes in mitochondrial ultrastructure, cellular ATP, ion and water content following injury of Ehrlich ascites tumor cells. Virchows Arch B Cell Pathol. 1974;15(4):267–277. doi: 10.1007/BF02889343. [DOI] [PubMed] [Google Scholar]
- Leaf A. Cell swelling. A factor in ischemic tissue injury. Circulation. 1973 Sep;48(3):455–458. doi: 10.1161/01.cir.48.3.455. [DOI] [PubMed] [Google Scholar]
- Leaf A. Regulation of intracellular fluid volume and disease. Am J Med. 1970 Sep;49(3):291–295. doi: 10.1016/s0002-9343(70)80019-5. [DOI] [PubMed] [Google Scholar]
- Lerner E., Shug A. L., Elson C., Shrago E. Reversible inhibition of adenine nucleotide translocation by long chain fatty acyl coenzyme A esters in liver mitochondria of diabetic and hibernating animals. J Biol Chem. 1972 Mar 10;247(5):1513–1519. [PubMed] [Google Scholar]
- Macknight A. D., Leaf A. Regulation of cellular volume. Physiol Rev. 1977 Jul;57(3):510–573. doi: 10.1152/physrev.1977.57.3.510. [DOI] [PubMed] [Google Scholar]
- Mclean P., Gumaa K. A., Greenbaum A. L. Long chain acyl CoAs, adenine nucleotide translocase and the coordination of the redox states of the cytosolic and mitochondrial compartments. FEBS Lett. 1971 Oct 1;17(2):345–350. doi: 10.1016/0014-5793(71)80184-9. [DOI] [PubMed] [Google Scholar]
- Mergner W. J., Smith M. W., Trump B. F. Studies on the pathogenesis of ischemic cell injury. XI. P/O ratio and acceptor control. Virchows Arch B Cell Pathol. 1977 Nov 30;26(1):17–26. doi: 10.1007/BF02889532. [DOI] [PubMed] [Google Scholar]
- Mittnacht S., Jr, Sherman S. C., Farber J. L. Reversal of ischemic mitochondrial dysfunction. J Biol Chem. 1979 Oct 10;254(19):9871–9878. [PubMed] [Google Scholar]
- Nachbaur J., Colbeau A., Vignais P. M. Distribution of membrane-confined phospholipases A in the rat hepatocyte. Biochim Biophys Acta. 1972 Aug 9;274(2):426–446. doi: 10.1016/0005-2736(72)90189-7. [DOI] [PubMed] [Google Scholar]
- Nadler S., Goldfischer S. The intracellular release of lysosomal contents in macrophages that have ingested silica. J Histochem Cytochem. 1970 May;18(5):368–371. doi: 10.1177/18.5.368. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Poole-Wilson P. A., Williams A. Hypoxia and calcium. J Mol Cell Cardiol. 1979 Jul;11(7):683–706. doi: 10.1016/0022-2828(79)90381-x. [DOI] [PubMed] [Google Scholar]
- Newkirk J. D., Waite M. Identification of a phospholipase A1 in plasma membranes of rat liver. Biochim Biophys Acta. 1971 Feb 2;225(2):224–233. doi: 10.1016/0005-2736(71)90215-x. [DOI] [PubMed] [Google Scholar]
- Pande S. V., Blanchaer M. C. Reversible inhibition of mitochondrial adenosine diphosphate phosphorylation by long chain acyl coenzyme A esters. J Biol Chem. 1971 Jan 25;246(2):402–411. [PubMed] [Google Scholar]
- Papahadjopoulos D., Jacobson K., Nir S., Isac T. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim Biophys Acta. 1973 Jul 6;311(3):330–348. doi: 10.1016/0005-2736(73)90314-3. [DOI] [PubMed] [Google Scholar]
- Pfeiffer D. R., Schmid P. C., Beatrice M. C., Schmid H. H. Intramitochondrial phospholipase activity and the effects of Ca2+ plus N-ethylmaleimide on mitochondrial function. J Biol Chem. 1979 Nov 25;254(22):11485–11494. [PubMed] [Google Scholar]
- Rottem S., Yashouv J., Ne'eman Z., Razin S. Cholesterol in mycoplasma membranes. Composition, ultrastructure and biological properties of membranes from Mycoplasma mycoides var. capri cells adapted to grow with low cholesterol concentrations. Biochim Biophys Acta. 1973 Nov 16;323(4):495–508. doi: 10.1016/0005-2736(73)90158-2. [DOI] [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Schanne F. A., Pfau R. G., Farber J. L. Galactosamine-induced cell death in primary cultures of rat hepatocytes. Am J Pathol. 1980 Jul;100(1):25–38. [PMC free article] [PubMed] [Google Scholar]
- Shechter E., Letellier L., Gulik-Krzywicki G. Relations between structure and function in cytoplasmic membrane vesicles isolated from an Escherichia coli fatty-acid auxotroph. High-angle x-ray diffraction, freeze-etch electron microscopy and transport studies. Eur J Biochem. 1974 Nov 1;49(1):61–76. doi: 10.1111/j.1432-1033.1974.tb03811.x. [DOI] [PubMed] [Google Scholar]
- Shen A. C., Jennings R. B. Myocardial calcium and magnesium in acute ischemic injury. Am J Pathol. 1972 Jun;67(3):417–440. [PMC free article] [PubMed] [Google Scholar]
- Shug A. L., Shrago E., Bittar N., Folts J. D., Koke J. R. Acyl-CoA inhibition of adenine nucleotide translocation in ischemic myocardium. Am J Physiol. 1975 Mar;228(3):689–692. doi: 10.1152/ajplegacy.1975.228.3.689. [DOI] [PubMed] [Google Scholar]
- Shug A. L., Thomsen J. H., Folts J. D., Bittar N., Klein M. I., Koke J. R., Huth P. J. Changes in tissue levels of carnitine and other metabolites during myocardial ischemia and anoxia. Arch Biochem Biophys. 1978 Apr 15;187(1):25–33. doi: 10.1016/0003-9861(78)90003-6. [DOI] [PubMed] [Google Scholar]
- Shug A., Lerner E., Elson C., Shrago E. The inhibition of adenine nucleotide translocase activity by oleoyl CoA and its reversal in rat liver mitochondria. Biochem Biophys Res Commun. 1971 May 7;43(3):557–563. doi: 10.1016/0006-291x(71)90650-4. [DOI] [PubMed] [Google Scholar]
- Speth V., Wunderlich F. Membranes of Tetrahymena. II. Direct visualization of reversible transitions in biomembrane structure induced by temperature. Biochim Biophys Acta. 1973 Feb 16;291(3):621–628. doi: 10.1016/0005-2736(73)90467-7. [DOI] [PubMed] [Google Scholar]
- Summers W. K., Jamison R. L. The no reflow phenomenon in renal ischemia. Lab Invest. 1971 Dec;25(6):635–643. [PubMed] [Google Scholar]
- Trump B. F., Laiho K. U. Studies of cellular recovery from injury. I. Recovery from anoxia in Ehrlich ascites tumor cells. Lab Invest. 1975 Dec;33(6):706–711. [PubMed] [Google Scholar]
- Verkleij A. J., Ververgaert P. H., van Deenen L. L., Elbers P. F. Phase transitions of phospholipid bilayers and membranes of Acholeplasma laidlawii B visualized by freeze fracturing electron microscopy. Biochim Biophys Acta. 1972 Nov 2;288(2):326–332. doi: 10.1016/0005-2736(72)90253-2. [DOI] [PubMed] [Google Scholar]
- Vogt M. T., Farber E. On the molecular pathology of ischemic renal cell death. Reversible and irreversible cellular and mitochondrial metabolic alterations. Am J Pathol. 1968 Jul;53(1):1–26. [PMC free article] [PubMed] [Google Scholar]
- Waite M., Sisson P. Partial purification and characterization of the phospholipase A 2 from rat liver mitochondria. Biochemistry. 1971 Jun 8;10(12):2377–2383. doi: 10.1021/bi00788a031. [DOI] [PubMed] [Google Scholar]
- Weglicki W. B., Waite M., Sisson P., Shohet S. B. Myocardial phospholipase A of microsomal and mitochondrial fractions. Biochim Biophys Acta. 1971 May 4;231(3):512–519. doi: 10.1016/0005-2760(71)90119-6. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Zurier R. B., Spieler P. J., Goldstein I. M. Mechanisms of lysosomal enzyme release from leukocytes exposed to immune complexes and other particles. J Exp Med. 1971 Sep 1;134(3 Pt 2):149s–165s. [PubMed] [Google Scholar]
- Whalen D. A., Jr, Hamilton D. G., Ganote C. E., Jennings R. B. Effect of a transient period of ischemia on myocardial cells. I. Effects on cell volume regulation. Am J Pathol. 1974 Mar;74(3):381–397. [PMC free article] [PubMed] [Google Scholar]
- Whitmer J. T., Idell-Wenger J. A., Rovetto M. J., Neely J. R. Control of fatty acid metabolism in ischemic and hypoxic hearts. J Biol Chem. 1978 Jun 25;253(12):4305–4309. [PubMed] [Google Scholar]
- Wildenthal K., Decker R. S., Poole A. R., Griffin E. E., Dingle J. T. Sequential lysosomal alterations during cardiac ischemia. I. Biochemical and immunohistochemical changes. Lab Invest. 1978 Jun;38(6):656–661. [PubMed] [Google Scholar]
- Wildenthal K. Lysosomal alterations in ischemic myocardium: result or cause of myocellular damage? J Mol Cell Cardiol. 1978 Jul;10(7):595–603. doi: 10.1016/s0022-2828(78)80001-7. [DOI] [PubMed] [Google Scholar]
- Willms-Kretschmer K., Majno G. Ischemia of the skin. Electron microscopic study of vascular injury. Am J Pathol. 1969 Mar;54(3):327–353. [PMC free article] [PubMed] [Google Scholar]
- Wunderlich F., Wallach D. F., Speth V., Fischer H. Differential effects of temperature on the nuclear and plasma membranes of lymphoid cells. A study by freeze-etch electron microscopy. Biochim Biophys Acta. 1974 Nov 27;373(1):34–43. doi: 10.1016/0005-2736(74)90102-3. [DOI] [PubMed] [Google Scholar]
- van den Bosch H. Phosphoglyceride metabolism. Annu Rev Biochem. 1974;43(0):243–277. doi: 10.1146/annurev.bi.43.070174.001331. [DOI] [PubMed] [Google Scholar]


