Abstract
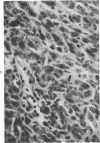
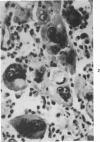
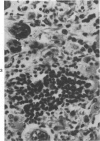
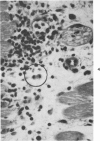
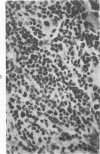
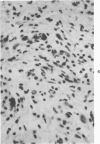
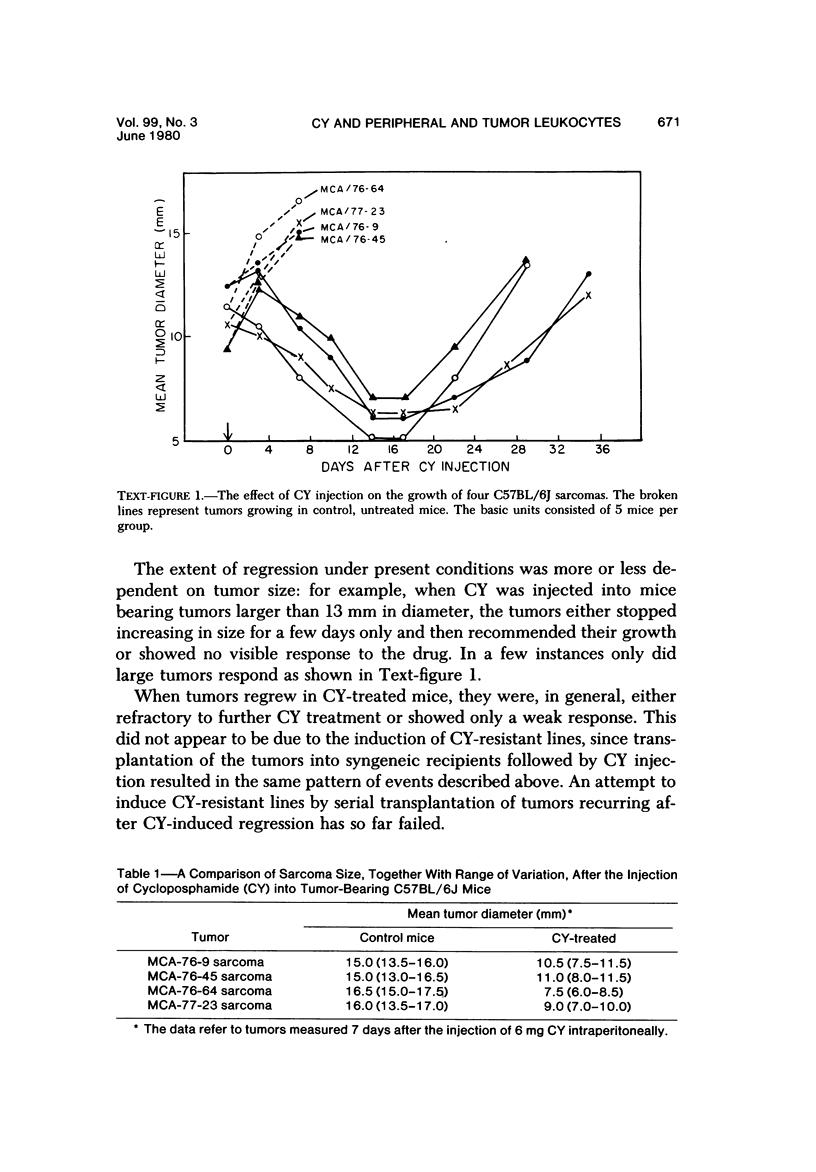
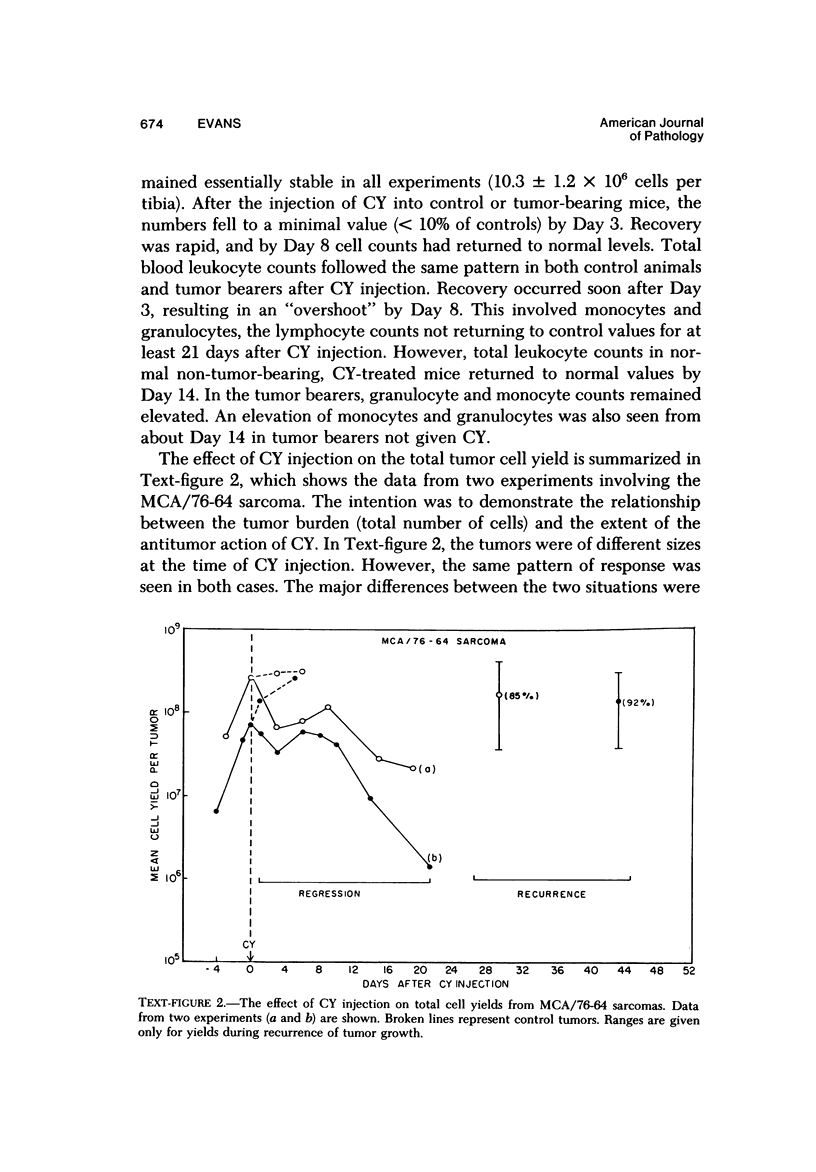
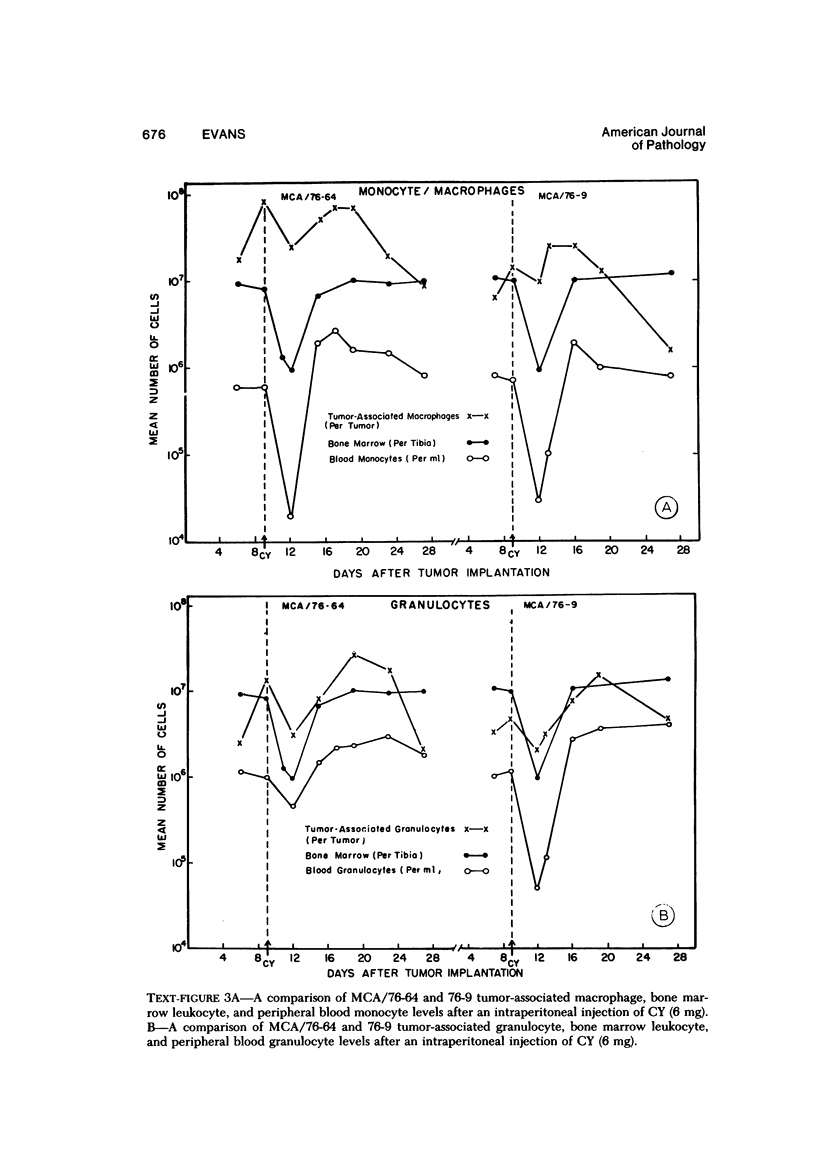
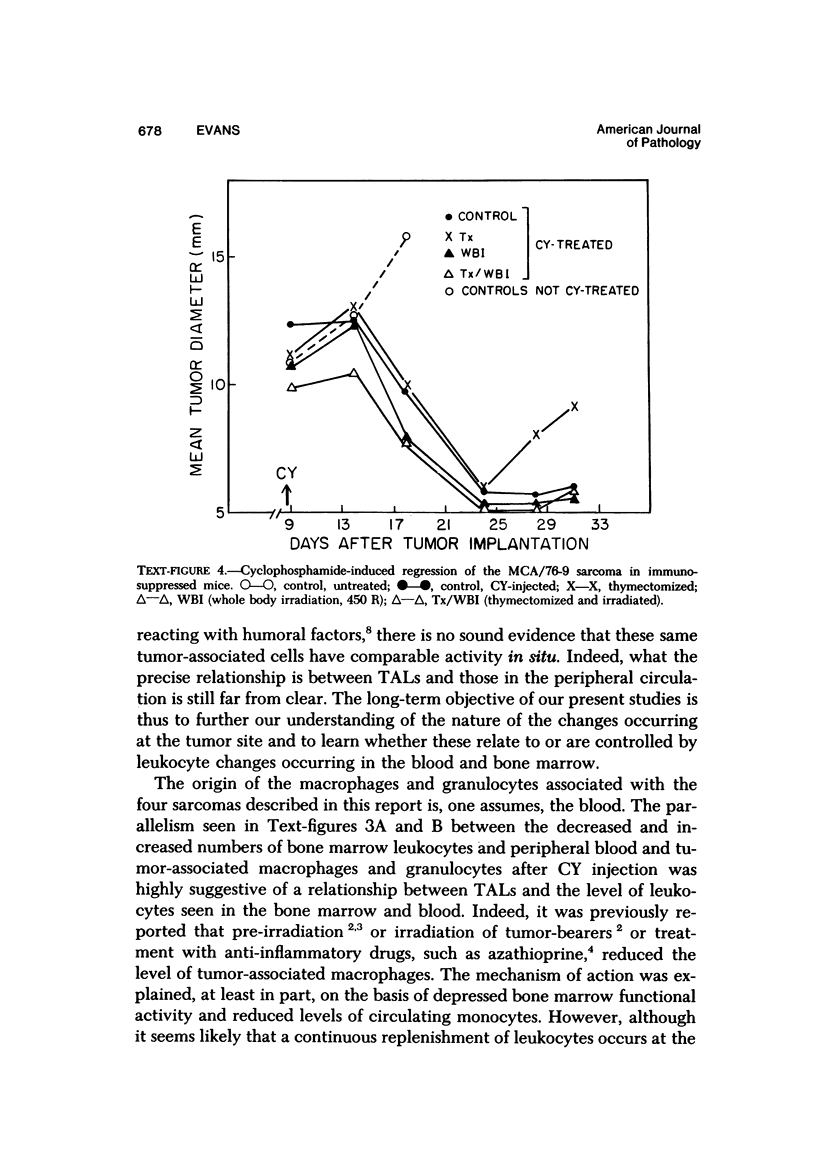
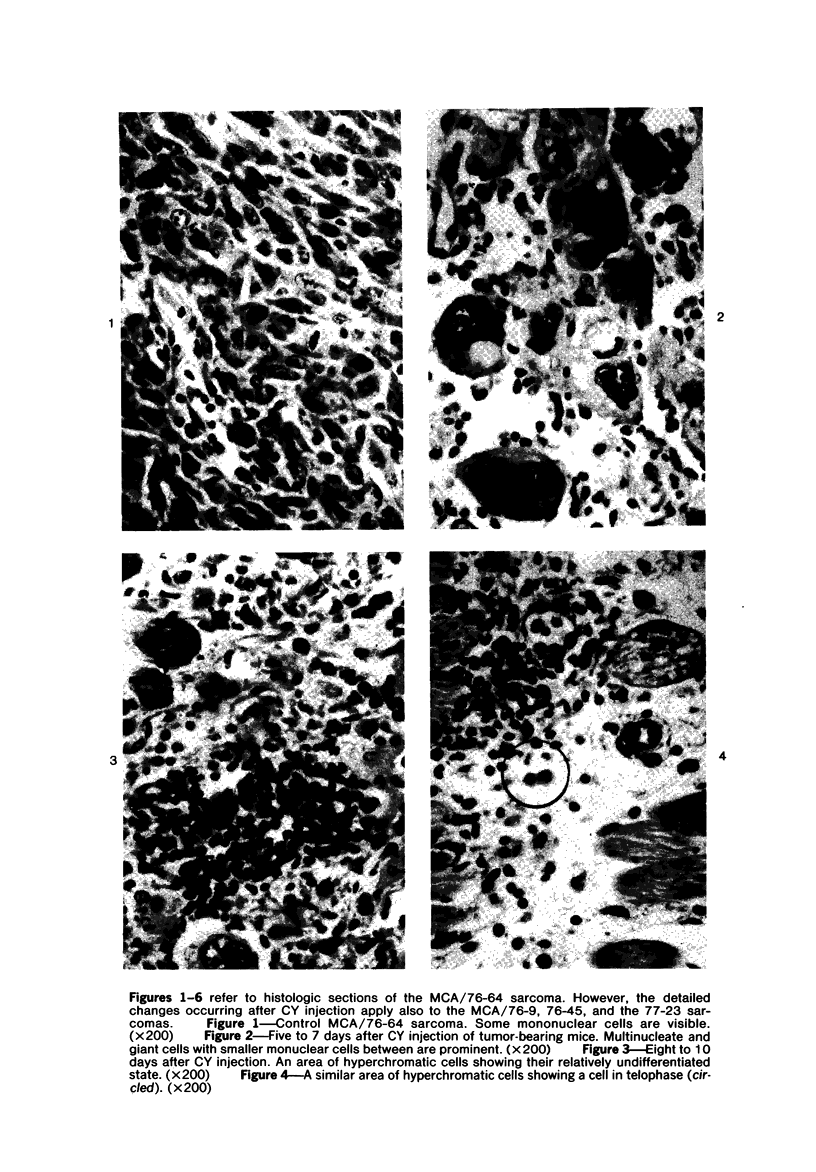
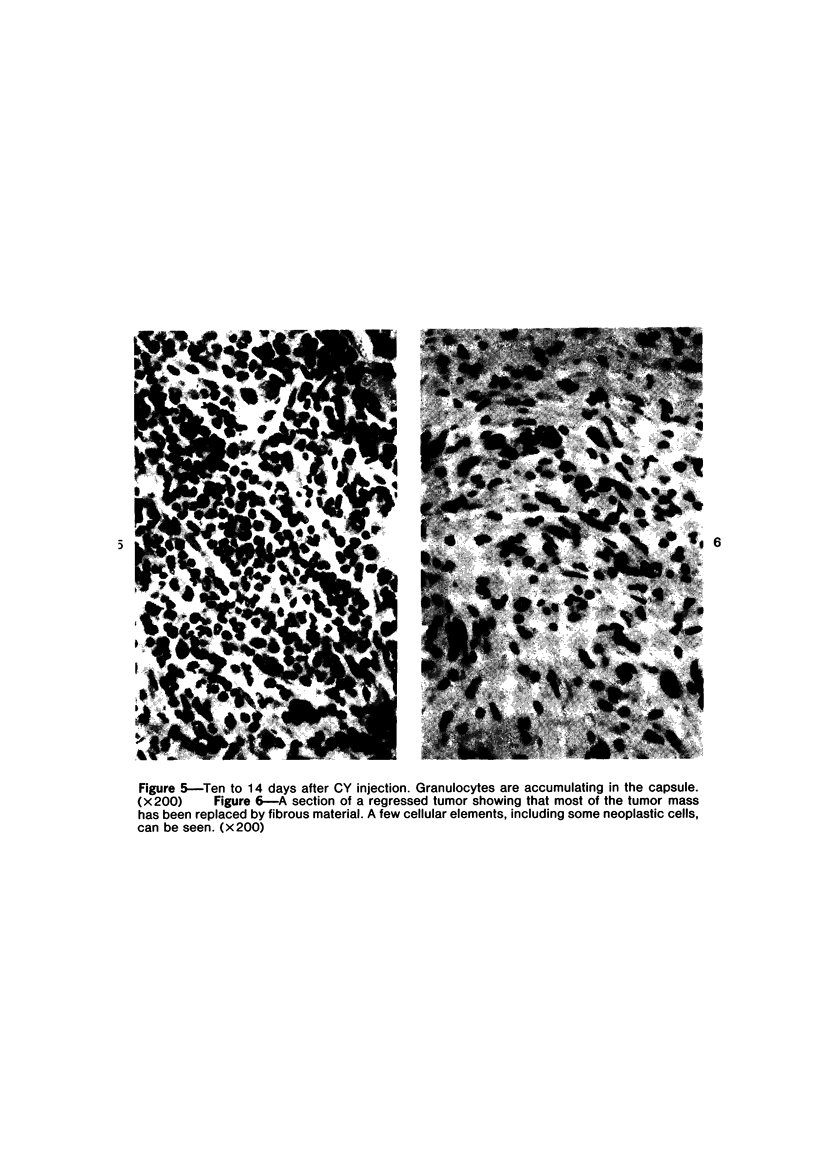
The injection of cyclophosphamide (CY) into C57BL/6J mice bearing intramuscularly transplanted, MCA-induced tumors (MCA/76-9, 76-64, 76-45, and 77-23) resulted in reproducible sequence of events involving tumor regression that was followed by recurrence in all but a few cases. Regression itself occurred whether tumors were immunogenic or not and whether tumors were growing in immunocompetent of immunosuppressed mice. Permanent CY-induced regressions, however, were rare under any conditions. The drug induced well-defined changes in the number of bone marrow and blood leukocytes. By 8 days after drug injecion, control or tumor-bearing mice showed normal bone marrow counts but elevated peripheral blood leukocyte counts. The latter involved only monocytes and granulocytes, lymphocytes requiring more than 21 days on return to control values. Tumor bearers not injected with CY also showed elevated blood monocyte and granulocyte numbers. After CY injection of mice, tumors showed distinctive histologic changes that were common for all four sarcomas. Within 3 days of injection, neoplastic cells showed evidence of damage, and mononuclear cells became prominent thoughout the tumor mass. By 7 days, there were many giant cells and polykaryons, which progressively decreased, so that by Days 10-14 few were discernible. At this time, the bulk of the tumor mass consisted of mononuclear cells having the morphologic characteristics of macrophages. However, between days 7 and 10 well-defined hyperchromatic areas could be seen peripherally in the capsule or the muscle into which the tumor cells were originally implanted. These areas consisted of relatively undifferentiated cell types, with which cells of the granulocyte series were often associated. By Day 14 these regions were packed with granulocytes. Regression usually stopped between Days 14 and 21, at which time the residual tumor mass consisted of birefringent, fibrous tissue containing relatively few cellular elements. Between 21 and 28 days, tumor recurrence was evident in most cases. The histologic changes were quantified by disaggregating the tumors with enzymes before and after CY injection. A good correlation was obtained between histologic appearance and the numbers and types of cells obtained at defined times. Both tumor-associated macrophage and granulocyte counts showed an increase over the first 10 days after CY injection. Frequently, both cell types declined numerically over the first 3 days in parallel with blood monocyte and granulocyte counts and with bone marrow cell counts. The decline depended to large extent on the total number of cells associated with the tumor mass and the overall effect exerted by CY on these cells. Intratumor macrophage numbers reached a peak by Day 8, whereas granulocytes did not reach maximum values until Day 10. Subsequently, both cell types decreased in number until recurrent tumor growth became apparent. These findings are discussed in terms of the possible relationship between peripheral and tumor-associated leukocytes and in terms of the functions of the latter during CY-induced tumor regression.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burkitt D. P. Host defence mechanisms in Burkitt's lymphoma and Kaposi's sarcoma: the clinical evidence. Br Med J. 1970 Nov 14;4(5732):424–426. doi: 10.1136/bmj.4.5732.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles S. A., Alexander P. Macrophage content of tumours in relation to metastatic spread and host immune reaction. Nature. 1974 Aug 23;250(5468):667–669. doi: 10.1038/250667a0. [DOI] [PubMed] [Google Scholar]
- Evans R. Effect of X-irradiation on host-cell infiltration and growth of a murine fibrosarcoma. Br J Cancer. 1977 May;35(5):557–566. doi: 10.1038/bjc.1977.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. Failure to relate the anti-tumour action of cyclophosphamide with the immunogenicity of two murine fibrosarcomas. Int J Cancer. 1978 May 15;21(5):611–616. doi: 10.1002/ijc.2910210511. [DOI] [PubMed] [Google Scholar]
- Evans R. Host cells in transplanted murine tumors and their possible relevance to tumor growth. J Reticuloendothel Soc. 1979 Oct;26(4):427–437. [PubMed] [Google Scholar]
- Evans R., Madison L. D., Eidlen D. M. Cyclophosphamide-induced changes in the cellular composition of a methylcholanthrene-induced tumor and their relation to bone marrow and blood leukocyte levels. Cancer Res. 1980 Feb;40(2):395–402. [PubMed] [Google Scholar]
- Evans R. The effect of azathioprine on host cell infiltration and growth of a murine fibrosarcoma. Int J Cancer. 1977 Jul 15;20(1):120–128. doi: 10.1002/ijc.2910200119. [DOI] [PubMed] [Google Scholar]
- Gill H. K., Liew F. Y. Regulation of delayed-type hypersensitivity. III. Effect of cyclophosphamide on the suppressor cells for delayed-type hypersensitivity to sheep erythrocytes in mice. Eur J Immunol. 1978 Mar;8(3):172–176. doi: 10.1002/eji.1830080306. [DOI] [PubMed] [Google Scholar]
- Haskill J. S., Yamamura Y., Radov L., Parthenais E. Discussion paper: are peripheral and in situ tumor immunity related? Ann N Y Acad Sci. 1976;276:373–380. doi: 10.1111/j.1749-6632.1976.tb41662.x. [DOI] [PubMed] [Google Scholar]
- Katz S. I., Parker D., Turk J. L. B-cell suppression of delayed hypersensitivity reactions. Nature. 1974 Oct 11;251(5475):550–551. doi: 10.1038/251550a0. [DOI] [PubMed] [Google Scholar]
- Mathé G., Halle-Pannenko O., Bourut C. Immune manipulation by BCG administered before or after cyclophosphamide for chemo-immunotherapy of L1210 leukaemia. Eur J Cancer. 1974 Oct;10(10):661–666. doi: 10.1016/0014-2964(74)90005-x. [DOI] [PubMed] [Google Scholar]
- Miller R. G., Shcilling R. M., Phillips R. A. Requirement for non T-cells in the generation of cytotoxic T lymphocytes (CTL) in vitro. II. Characterization of the active cells in the spleen of nude mice. J Immunol. 1977 Jan;118(1):166–174. [PubMed] [Google Scholar]
- Moore M., Williams D. E. Contribution of host immunity to cyclophosphamide therapy of a chemically-induced murine sarcoma. Int J Cancer. 1973 Mar 15;11(2):358–368. doi: 10.1002/ijc.2910110213. [DOI] [PubMed] [Google Scholar]
- Neta R., Salvin S. B. T and B lymphocytes in the regulation of delayed hypersensitivity. J Immunol. 1976 Nov;117(5 PT2):2014–2020. [PubMed] [Google Scholar]
- Radov L. A., Haskill J. S., Korn J. H. Host immune potentiation of drug responses to a murine mammary adenocarcinoma. Int J Cancer. 1976 Jun 15;17(6):773–779. doi: 10.1002/ijc.2910170613. [DOI] [PubMed] [Google Scholar]
- Snodgrass M. J., Hanna M. G., Jr Ultrastructural studies of histiocyte-tumor cell interactions during tumor regression after intralesional injection of Mycobacterium bovis. Cancer Res. 1973 Apr;33(4):701–716. [PubMed] [Google Scholar]
- Steele G., Pierce G. E. Effects of cyclophosphamide on immunity against chemically-induced syngeneic murine sarcomas. Int J Cancer. 1974 Apr 15;13(4):572–578. doi: 10.1002/ijc.2910130417. [DOI] [PubMed] [Google Scholar]
- Ziegler J. L., Morrow R. H., Jr, Fass L., Kyalwazi S. K., Carbone P. P. Treatment of Burkitt's tumor with cyclophosphamide. Cancer. 1970 Aug;26(2):474–484. doi: 10.1002/1097-0142(197008)26:2<474::aid-cncr2820260232>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]