Abstract
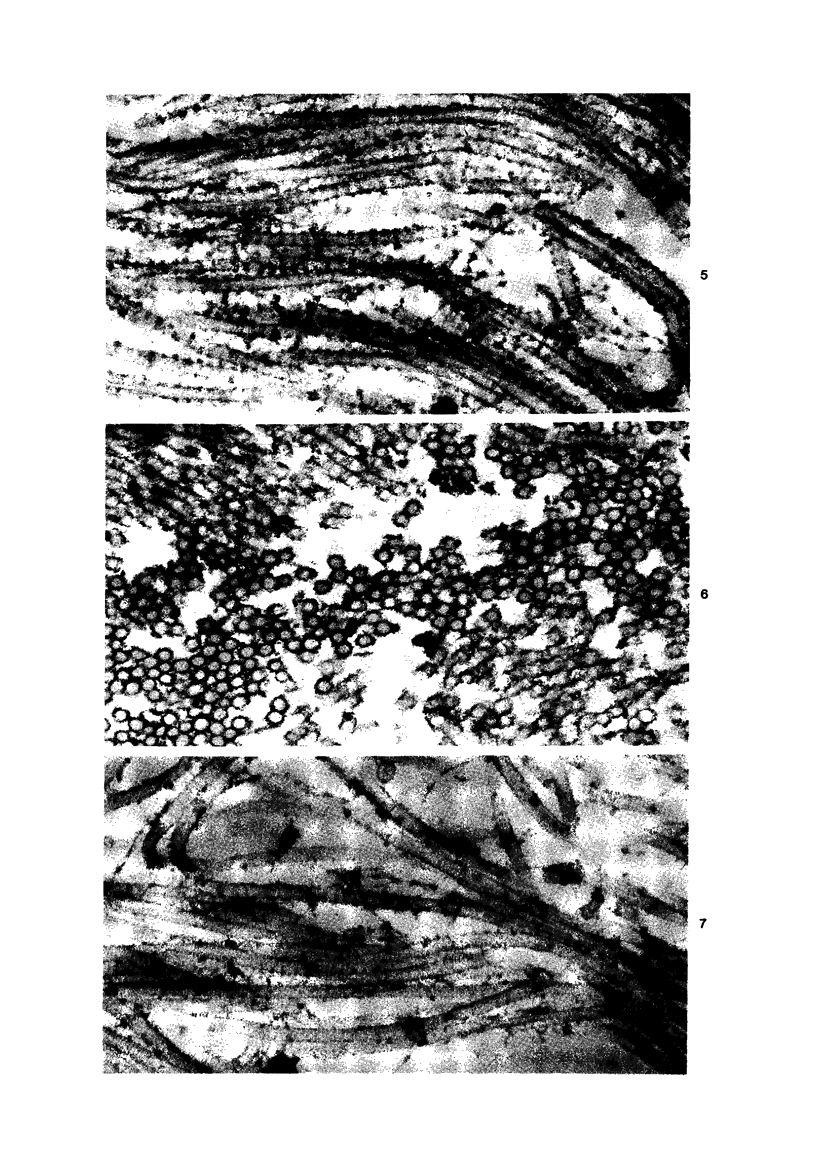
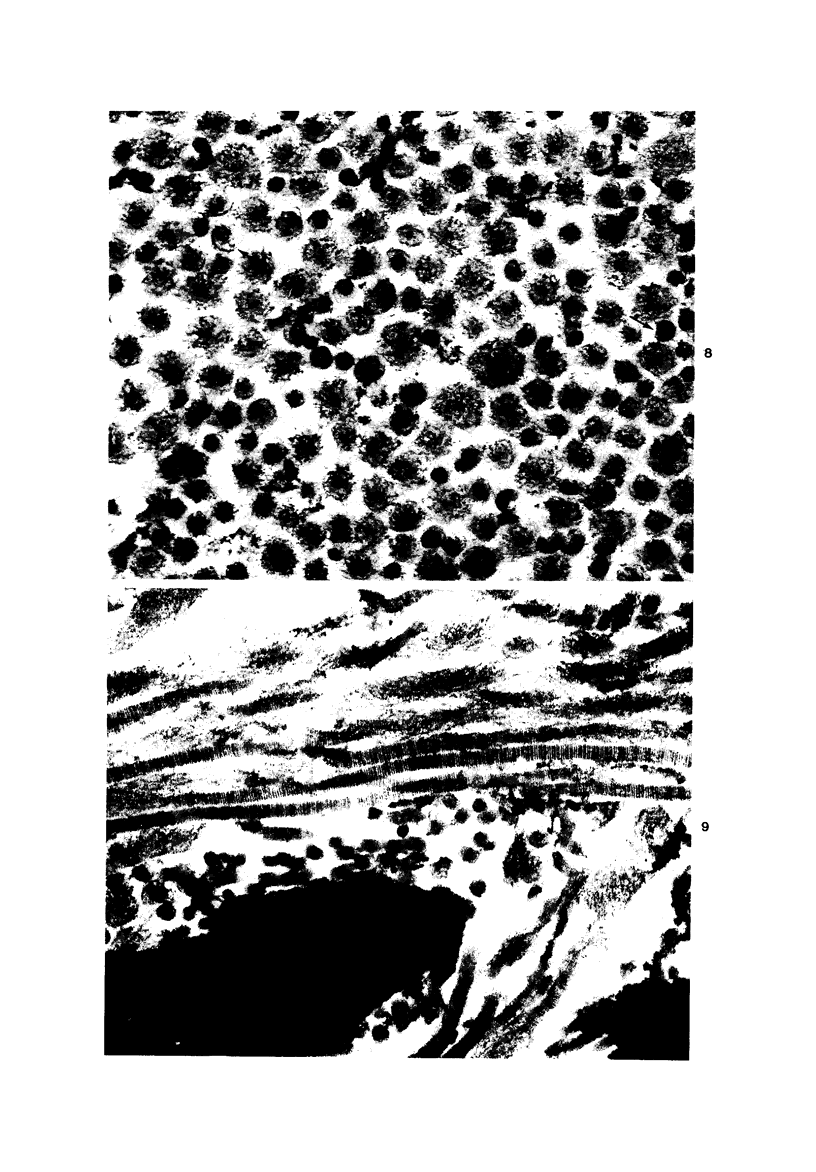
Injection of a solution of 30% urea in acetate buffer at pH 4.0 into the stroma of the uterine cervix results in a marked change in the mechanical properties of the tissue with subsequent easy dilatation. This study analyzes the changes in the collagenous matrix of the cervix by ultrastructural and biochemical methods. Collagen fibrils in urea-treated sections of the cervix are swollen and unravelled, showing spiral configuration of subunits in both cross-sections and longitudinal sections. The regular localization of ruthenium-red-positive material is absent in urea-treated tissues. Chemical analysis of incubated cervical tissues shows a reduction of the total glycosaminoglycans and of dermatan sulfate with release of the latter into the medium. It is suggested that urea dissociates intercollagen linkages by solubilizing a certain glycosaminoglycan, possibly dermatan sulfate. After this solubilization the collagen fibril is prompted to unwind, resolving the collagen microfibrils, which appear to be organized in a spiral fashion.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bryant W. M., Greenwell J. E., Weeks P. M. Alterations in collagen organization during dilatation of the cervix uteri. Surg Gynecol Obstet. 1968 Jan;126(1):27–39. [PubMed] [Google Scholar]
- Danforth D. N., Veis A., Breen M., Weinstein H. G., Buckingham J. C., Manalo P. The effect of pregnancy and labor on the human cervix: changes in collagen, glycoproteins, and glycosaminoglycans. Am J Obstet Gynecol. 1974 Nov 1;120(5):641–651. doi: 10.1016/0002-9378(74)90608-5. [DOI] [PubMed] [Google Scholar]
- Droegemueller W., Chvapil M., Vining J., Whitaker L., Christian C. D. Urea and dilatation of the cervix. Am J Obstet Gynecol. 1978 Dec 1;132(7):775–782. doi: 10.1016/s0002-9378(78)80012-x. [DOI] [PubMed] [Google Scholar]
- GORDON J. A., JENCKS W. P. The relationship of structure to the effectiveness of denaturing agents for proteins. Biochemistry. 1963 Jan-Feb;2:47–57. doi: 10.1021/bi00901a011. [DOI] [PubMed] [Google Scholar]
- GROSS J., KIRK D. The heat precipitation of collagen from neutral salt solutions: some rate-regulating factors. J Biol Chem. 1958 Aug;233(2):355–360. [PubMed] [Google Scholar]
- Gelman R. A., Blackwell J. Interactions between mucopolysaccharides and cationic polypeptides in aqueous solution: hyaluronic acid, heparitin sulfate, and keratan sulfate. Biopolymers. 1974 Jan;13(1):139–156. doi: 10.1002/bip.1974.360130109. [DOI] [PubMed] [Google Scholar]
- Highton T. C., Myers D. B., Rayns D. G. The intercellular spaces of synovial tissue. N Z Med J. 1968 Mar;67(429):315–325. [PubMed] [Google Scholar]
- Lillie J. H., MacCallum D. K., Scaletta L. J., Occhino J. C. Collagen structure: evidence for a helical organization of the collagen fibril. J Ultrastruct Res. 1977 Feb;(2):134–143. doi: 10.1016/s0022-5320(77)90025-9. [DOI] [PubMed] [Google Scholar]
- Mier P. D., Wood M. A simplified technique for the analysis of tissue acid mucopolysaccharides. Clin Chim Acta. 1969 Apr;24(1):105–110. doi: 10.1016/0009-8981(69)90146-6. [DOI] [PubMed] [Google Scholar]
- Oegema T. R., Jr, Laidlaw J., Hascall V. C., Dziewiatkowski D. D. The effect of proteoglycans on the formation of fibrils from collagen solutions. Arch Biochem Biophys. 1975 Oct;170(2):698–709. doi: 10.1016/0003-9861(75)90167-8. [DOI] [PubMed] [Google Scholar]
- PEACOCK E. E., Jr, BIGGERS P. W. Measurement and significance of heat-labile and urea-sensitive cross-linking mechanisms in collagen of healing wounds. Surgery. 1963 Jul;54:144–151. [PubMed] [Google Scholar]
- Ruggeri A., Benazzo F., Reale E. Collagen fibrils with straight and helicoidal microfibrils: a freeze-fracture and thin-section study. J Ultrastruct Res. 1979 Jul;68(1):101–108. doi: 10.1016/s0022-5320(79)90146-1. [DOI] [PubMed] [Google Scholar]
- Shetlar M. R., Shetlar C. L., Chien S. F., Linares H. A., Dobrkovsky M., Larson D. L. The hypertrophic scar. Hexosamine containing components of burn scars. Proc Soc Exp Biol Med. 1972 Feb;139(2):544–547. doi: 10.3181/00379727-139-36182. [DOI] [PubMed] [Google Scholar]