Abstract
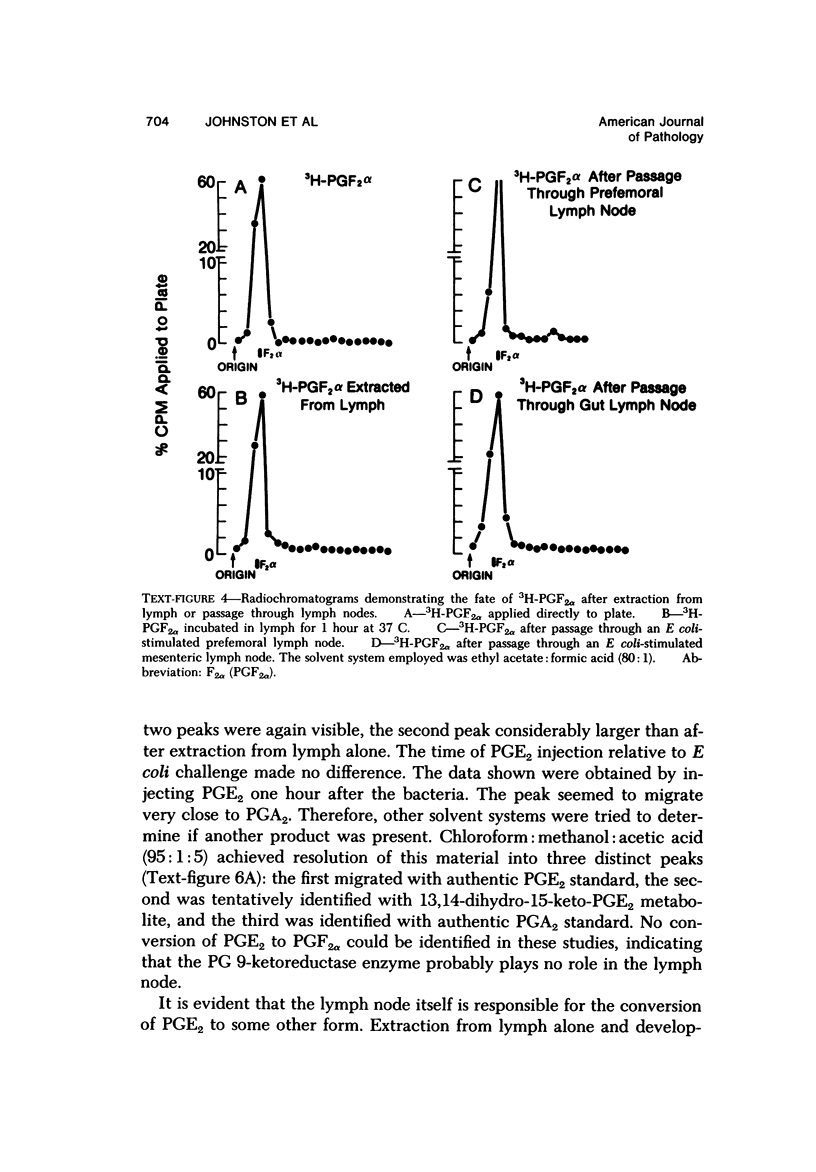
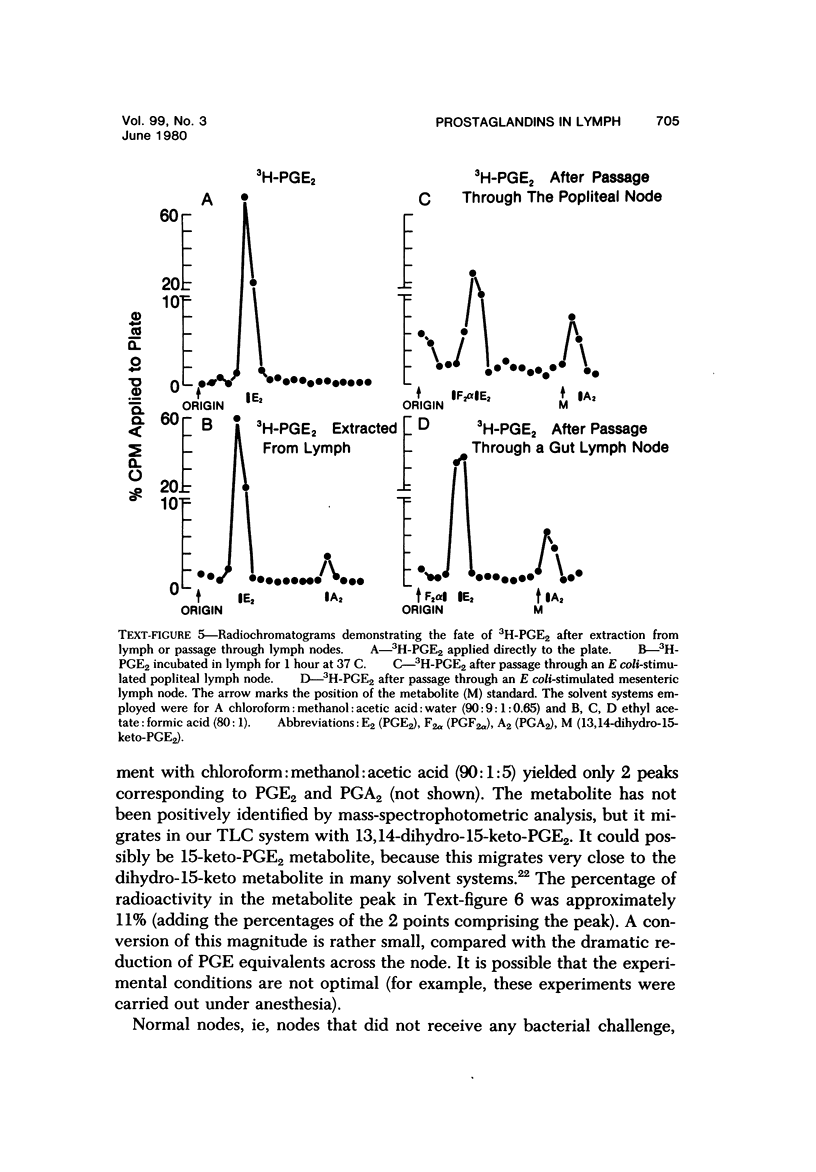
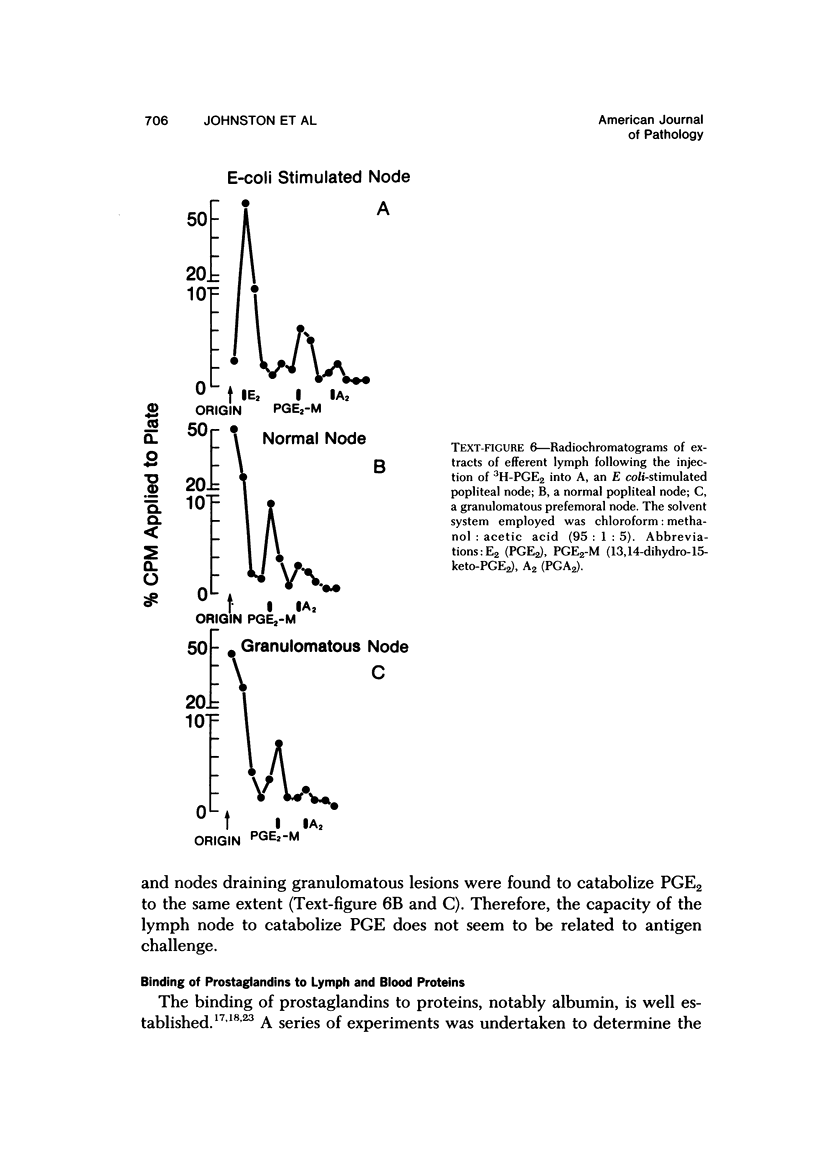
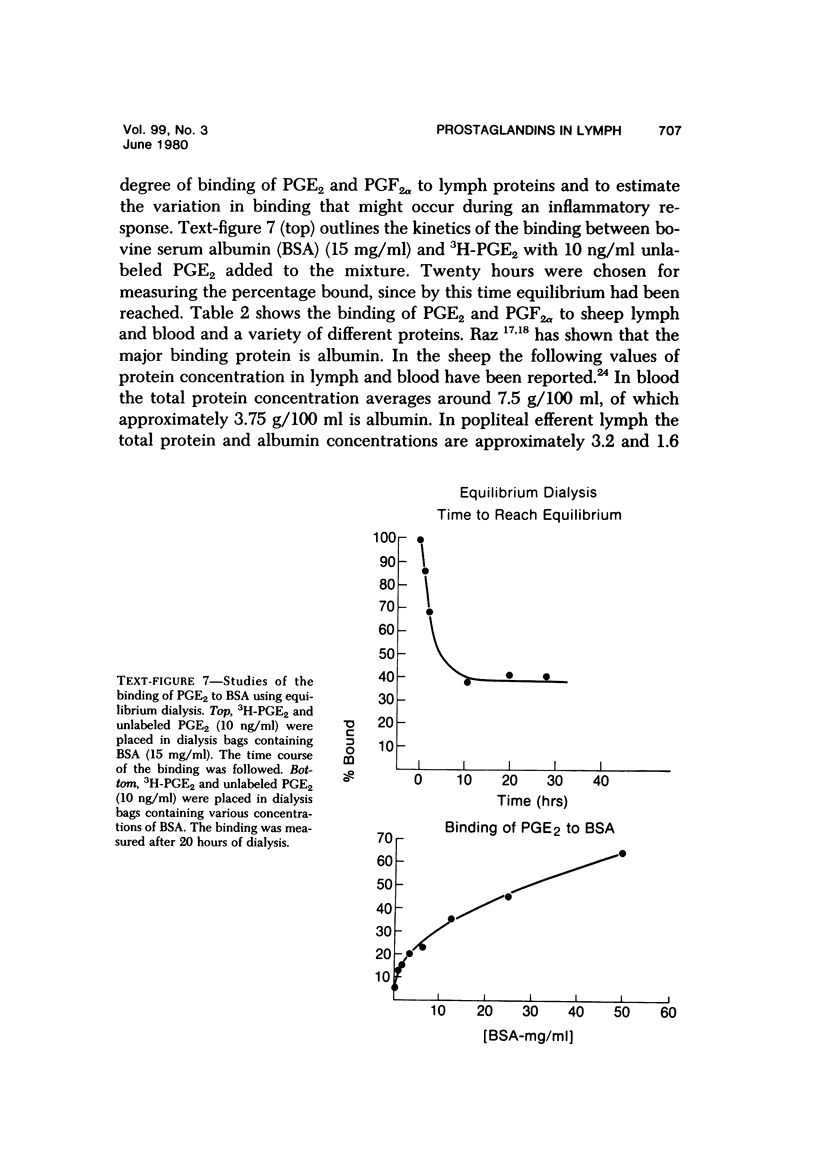
Arachidonic acid labeled with 14C was injected directly into lymph nodes that had been stimulated at various times with Escherichia coli. The efferent lymph was collected, and labeled catabolites were extracted and analyzed chromatographically. The pimary conversion product recovered was Prostaglandin E2 (PGE2), with the lesser products thromboxane, prostacyclin and prostaglandin F2α (PGF2α) also detected. When the efferent lymph was analyzed by radioimmunoassay after subcutaneous injectino of E coli into the hock, PGE and PGF levels rapidly increased, reached the highest levels in the first 10 hours, and then returned to normal by 24 hours. When the afferent lymph plasma draining inflammatory sites was compared directly with efferent lymph, PGF levels were similar, but the PGE level was always several times higher in the afferent lymph. To examine the catabolism of PG, either 3,H-PGF2ά of 3H-PGE2 was injected into the node, and the efferent lymph plasma was analyzed. No conversion of PGF2α to other products was found. In contrast, catabolic products of PGE2 were detected. With the use of equilibrium dialysis techniques, the binding of PGE2 and PGF2α to proteins in lymph and to bovine serum albumin (BSA), human serum albumin (HSA), and BSA stripped of its fatty acids was established. The binding to lymph proteins correlated with the albumin concentrations in the lymph. This albumin binging probably facilitated the retention and transport of PG in the lymph. PG appears in the lymph at a time corresponding to the uptake and processing of antigen by the node and near the time when lymphokines are detected in lymph and could modulate several steps in the immune response. The PGE detectable in the lymph draining an inflammatory site may play a role in the modulation of blood flow.
Full text
PDF



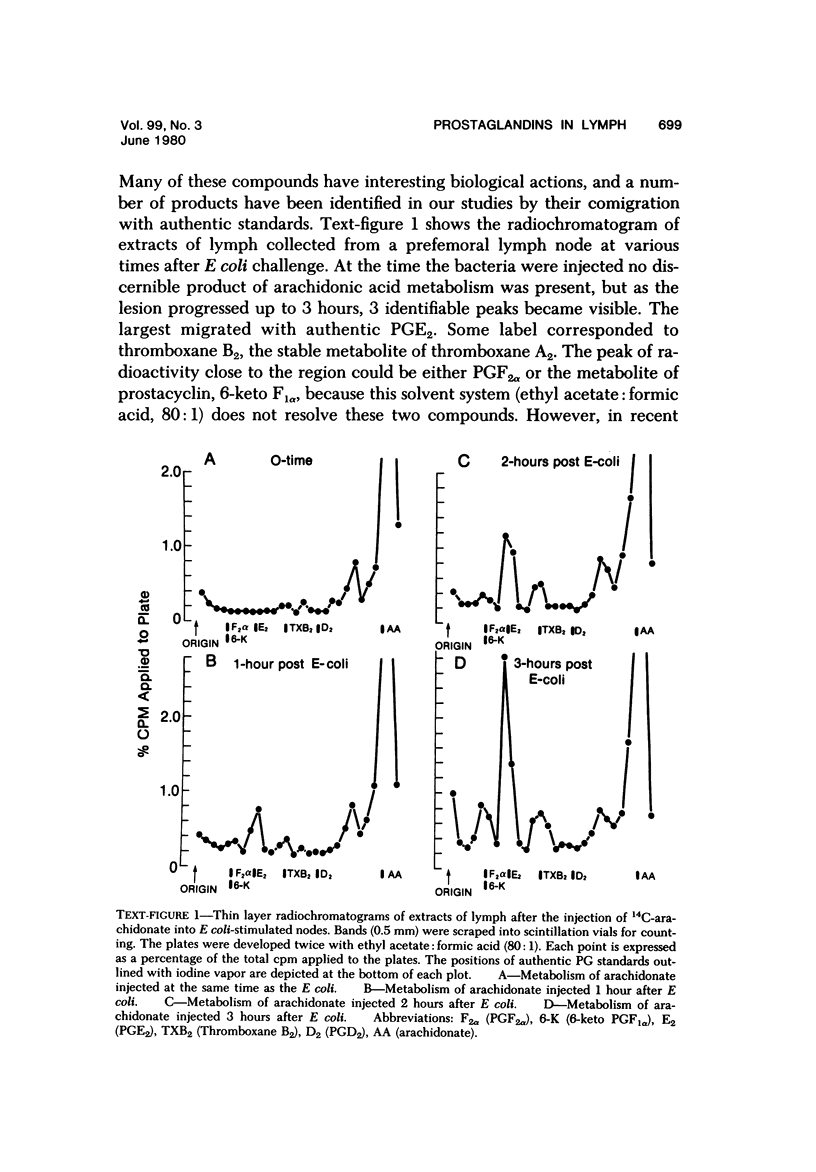
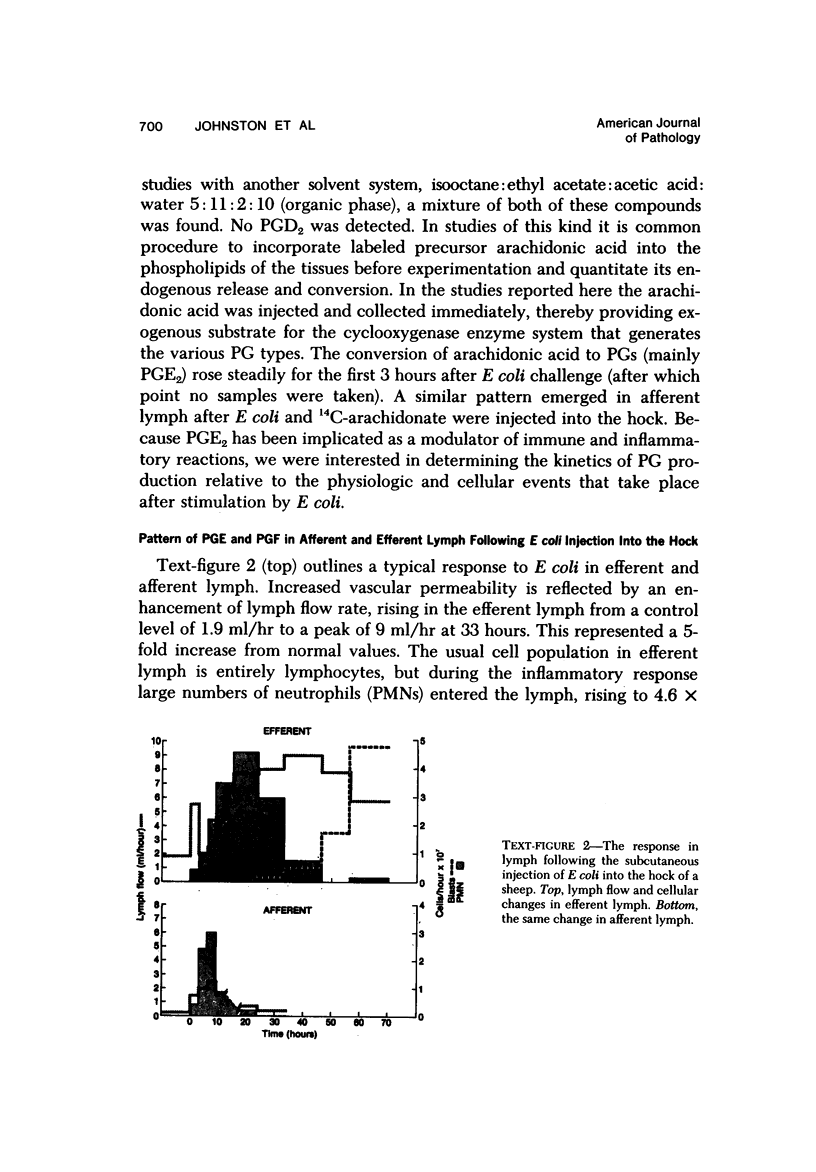
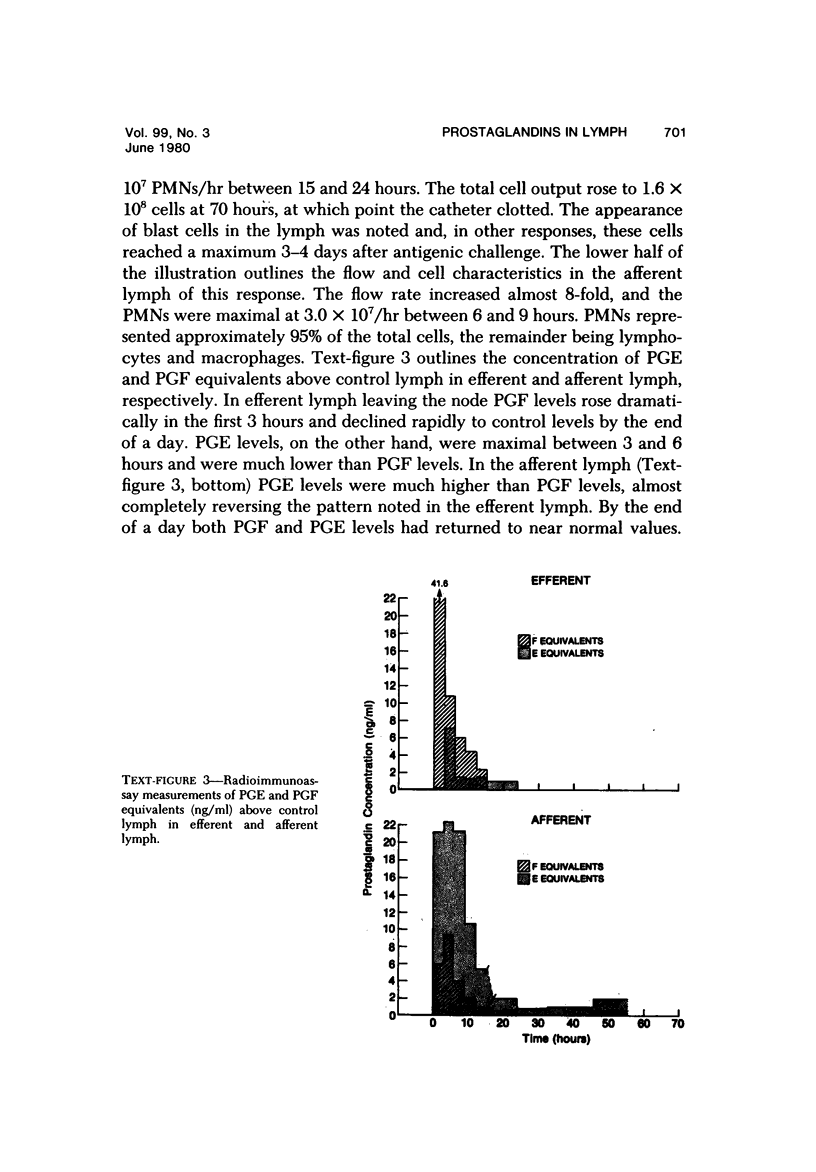







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. M., Lattimer N., Moncada S., Vane J. R. Comparison of the vasodepressor effects of prostacyclin and 6-oxo-prostaglandin F1alpha with those of prostaglandin E2 in rats and rabbits. Br J Pharmacol. 1978 Jan;62(1):125–130. doi: 10.1111/j.1476-5381.1978.tb07014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla J., Carson G. D., Challis J. R. Conversion of PGE2 to PGF2alpha by fetal sheep blood. Prostaglandins. 1977 Nov;14(5):873–879. doi: 10.1016/0090-6980(77)90303-3. [DOI] [PubMed] [Google Scholar]
- Bonta I. L., Parnham M. J. Prostaglandins and chronic inflammation. Biochem Pharmacol. 1978;27(12):1611–1623. doi: 10.1016/0006-2952(78)90169-7. [DOI] [PubMed] [Google Scholar]
- Crutchley D. J., Piper P. J., Seale J. P. The nature of prostaglandin-like substances released from guinea-pig lung in anaphylaxis. Eur J Pharmacol. 1977 Aug 15;44(4):319–323. doi: 10.1016/0014-2999(77)90305-3. [DOI] [PubMed] [Google Scholar]
- Crutchley D. J., Piper P. J. The behaviour of the pulmonary metabolites of prostaglandins in several simple thin-layer chromatography and bioassay systems. Prostaglandins. 1976 Jun;11(6):987–997. doi: 10.1016/0090-6980(76)90007-1. [DOI] [PubMed] [Google Scholar]
- English L., Morris B., Adams E. The modulating effects of lymph on the synthesis of immunoglobulins by lymphoblasts. Cell Immunol. 1977 Jan;28(1):90–100. doi: 10.1016/s0008-8749(77)80009-9. [DOI] [PubMed] [Google Scholar]
- Gerkens J. F., Friesinger G. C., Branch R. A., Shand D. G., Gerber J. G. A comparison of the pulmonary, renal and hepatic extractions of PGI2 and PGE2 - PGI2 a potential circulating hormone. Life Sci. 1978 May 22;22(20):1837–1842. doi: 10.1016/0024-3205(78)90601-x. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Selinger D. S., Messner R. P., Reed W. P. Effect of indomethacin in vivo on humoral and cellular immunity in humans. Infect Immun. 1978 Feb;19(2):430–433. doi: 10.1128/iai.19.2.430-433.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D., Bray M. A., Morley J. Control of lymphokine secretion by prostaglandins. Nature. 1976 Jul 29;262(5567):401–402. doi: 10.1038/262401a0. [DOI] [PubMed] [Google Scholar]
- Gueriguian F. L. Prostaglandin-macromolecule interactions. I. Noncovalent binding of prostaglandins A1, E1, F2alpha, and E2 by human and bovine serum albumins. J Pharmacol Exp Ther. 1976 May;197(2):391–401. [PubMed] [Google Scholar]
- HALL J. G., MORRIS B. The output of cells in lymph from the popliteal node of sheep. Q J Exp Physiol Cogn Med Sci. 1962 Oct;47:360–369. doi: 10.1113/expphysiol.1962.sp001620. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay J. B., Hobbs B. B., Johnston M. G., Movat H. Z. The role of hyperemia in cellular hypersensitivity reactions. Int Arch Allergy Appl Immunol. 1977;55(1-6):324–331. doi: 10.1159/000231943. [DOI] [PubMed] [Google Scholar]
- Hay J. B., Hobbs B. B. The flow of blood to lymph nodes and its relation to lymphocyte traffic and the immune response. J Exp Med. 1977 Jan 1;145(1):31–44. doi: 10.1084/jem.145.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay J. B., Morris B. Generation and selection of specific reactive cells by antigen. Br Med Bull. 1976 May;32(2):135–140. doi: 10.1093/oxfordjournals.bmb.a071345. [DOI] [PubMed] [Google Scholar]
- Johnston M. G., Hay J. B., Movat H. Z. Kinetics of prostaglandin production in various inflammatory lesions, measured in draining lymph. Am J Pathol. 1979 Apr;95(1):225–238. [PMC free article] [PubMed] [Google Scholar]
- Johnston M. G., Hay J. B., Movat H. Z. The modulation of enhanced vascular permeability by prostaglandins through alterations in blood flow (hyperemia). Agents Actions. 1976 Nov;6(6):705–711. doi: 10.1007/BF02026092. [DOI] [PubMed] [Google Scholar]
- Johnston M. G., Hay J. B., Movat H. Z. The role of prostaglandins in inflammation. Curr Top Pathol. 1979;68:259–287. doi: 10.1007/978-3-642-67311-5_10. [DOI] [PubMed] [Google Scholar]
- Levine L. Antibodies to pharmacologically active molecules: specificities and some applications of antiprostaglandins. Pharmacol Rev. 1973 Jun;25(2):293–307. [PubMed] [Google Scholar]
- Lichtenstein L. M., Gillespie E., Bourne H. R., Henney C. S. The effects of a series of prostaglandins on in vitro models of the allergic response and cellular immunity. Prostaglandins. 1972 Dec;2(6):519–528. doi: 10.1016/s0090-6980(72)80040-6. [DOI] [PubMed] [Google Scholar]
- Moncada S., Vane J. R. Unstable metabolites of arachidonic acid and their role in haemostasis and thrombosis. Br Med Bull. 1978 May;34(2):129–135. doi: 10.1093/oxfordjournals.bmb.a071482. [DOI] [PubMed] [Google Scholar]
- Muscoplat C. C., Rakich P. M., Thoen C. O., Johnson D. W. Enhancement of lymphocyte blastogenic and delayed hypersensitivity skin responses by indomethacin. Infect Immun. 1978 Jun;20(3):627–631. doi: 10.1128/iai.20.3.627-631.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Asciak C. R., Rangaraj G. Prostaglandin biosynthesis and catabolism in the lamb ductus arteriosus, aorta and pulmonary artery. Biochim Biophys Acta. 1978 Apr 28;529(1):13–20. doi: 10.1016/0005-2760(78)90098-x. [DOI] [PubMed] [Google Scholar]
- Pelus L. M., Strausser H. R. Prostaglandins and the immune response. Life Sci. 1977 Mar 15;20(6):903–913. doi: 10.1016/0024-3205(77)90274-0. [DOI] [PubMed] [Google Scholar]
- Piper P. J., Vane J. R., Wyllie J. H. Inactivation of prostaglandins by the lungs. Nature. 1970 Feb 14;225(5233):600–604. doi: 10.1038/225600a0. [DOI] [PubMed] [Google Scholar]
- Raz A. Interaction of prostaglandins with blood plasma proteins. Comparative binding of prostaglandins A 2 , F 2 and E 2 to human plasma proteins. Biochem J. 1972 Nov;130(2):631–636. doi: 10.1042/bj1300631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A. Interaction of prostaglandins with blood plasma proteins. I. Binding of prostaglandin E 2 to human plasma proteins and its effect on the physiological activity of prostaglandin E 2 in vitro and in vivo. Biochim Biophys Acta. 1972 Dec 8;280(4):602–613. [PubMed] [Google Scholar]
- Smith R. J. Modulation of phagocytosis by and lysosomal enzyme secretion from guinea-pig neutrophils: effect of nonsteroid anti-inflammatory agents and prostaglindins. J Pharmacol Exp Ther. 1977 Mar;200(3):647–657. [PubMed] [Google Scholar]
- Stockman G. D., Mumford D. M. The effect of prostaglandins on the in vitro blastogenic response of human peripheral blood lymphocytes. Exp Hematol. 1974;2(2):65–72. [PubMed] [Google Scholar]
- Webb D. R., Nowowiejski I. The role of prostaglandins in the control of the primary 19S immune response to sRBC. Cell Immunol. 1977 Sep;33(1):1–10. doi: 10.1016/0008-8749(77)90129-0. [DOI] [PubMed] [Google Scholar]
- Webb D. R., Osheroff P. L. Antigen stimulation of prostaglandin synthesis and control of immune responses. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1300–1304. doi: 10.1073/pnas.73.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]


