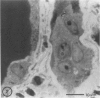
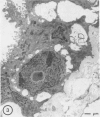
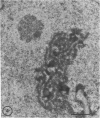
Abstract
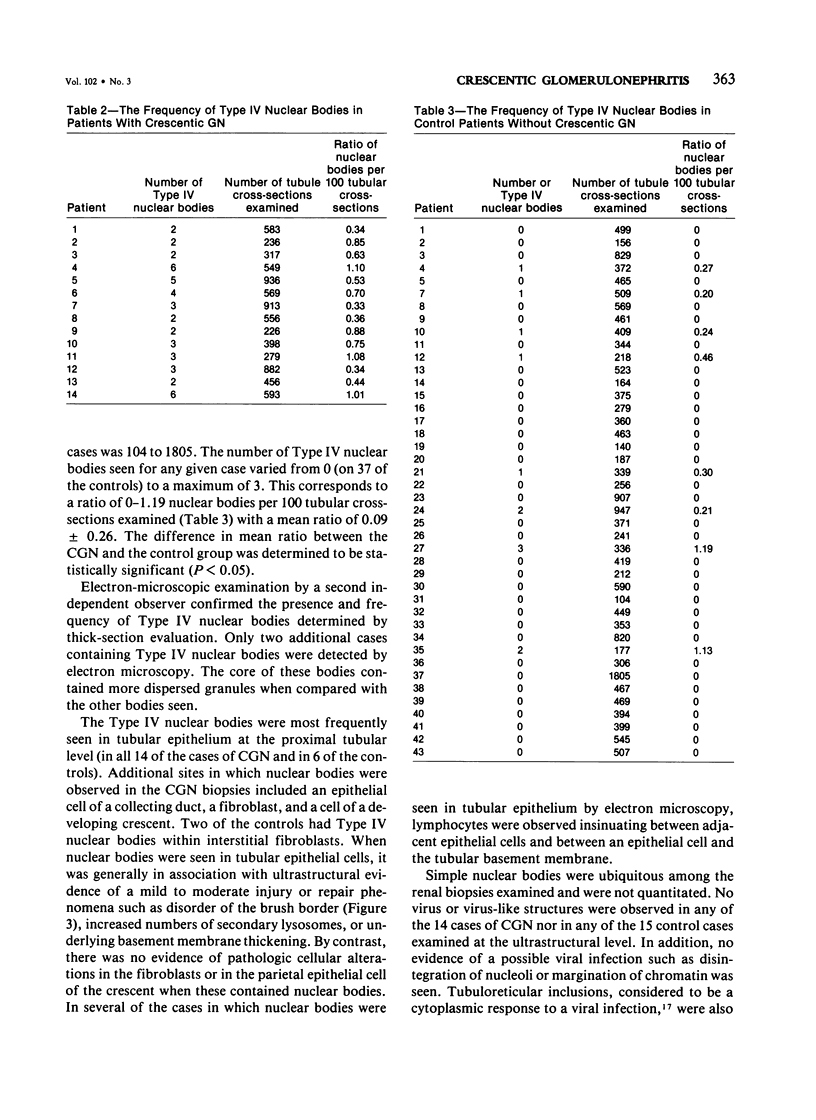
Type IV nuclear bodies were found in all renal biopsies obtained from 14 patients with crescentic glomerulonephritis (CGN) and in 8 of 43 control patients studied by light and electron microscopy. The mean ratio of Type IV nuclear bodies per 10 tubular cross-sections examined was 0.67 +/- 0.27 for the CGN cases, which was significantly different from 0.09 +/- 0.26 obtained for the controls (P less than 0.05). Type IV nuclear bodies were most commonly seen in the epithelial cells of proximal tubules but were also seen occasionally in interstitial fibroblasts and cells of a collecting duct and a developing crescent.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENOIT F. L., RULON D. B., THEIL G. B., DOOLAN P. D., WATTEN R. H. GOODPASTURE'S SYNDROME: A CLINICOPATHOLOGIC ENTITY. Am J Med. 1964 Sep;37:424–444. doi: 10.1016/0002-9343(64)90199-8. [DOI] [PubMed] [Google Scholar]
- BERNHARD W., FEBVRE H. L., CRAMER R. [Electron microscopic demonstration of a virus in cells infected in vitro by the polyoma agent]. C R Hebd Seances Acad Sci. 1959 Jul 20;249:483–485. [PubMed] [Google Scholar]
- Beirne G. J., Wagnild J. P., Zimmerman S. W., Macken P. D., Burkholder P. M. Idiopathic crescentic glomerulonephritis. Medicine (Baltimore) 1977 Sep;56(5):349–381. doi: 10.1097/00005792-197709000-00001. [DOI] [PubMed] [Google Scholar]
- Bouteille M., Kalifat S. R., Delarue J. Ultrastructural variations of nuclear bodies in human diseases. J Ultrastruct Res. 1967 Aug 30;19(5):474–486. doi: 10.1016/s0022-5320(67)80074-1. [DOI] [PubMed] [Google Scholar]
- Büttner D. W., Horstmann E. Das Sphaeridion, eine weit verbreitete Differenzierung des Karyoplasma. Z Zellforsch Mikrosk Anat. 1967;77(4):589–605. [PubMed] [Google Scholar]
- Büttner D. W., Horstmann E. Haben die Sphaeridien in den Zellkernen kranker Gewebe eine pathognomonische Bedeutung? Virchows Arch Pathol Anat Physiol Klin Med. 1967;343(2):142–163. [PubMed] [Google Scholar]
- Collan Y., Lähdevirta J. Electron microscopy of nephropathia epidemica cell nuclei in kidney biopsies. Acta Pathol Microbiol Scand A. 1979 Jan;87(1):71–77. doi: 10.1111/j.1699-0463.1979.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Dupuy-Coin A. M., Kalifat S. R., Bouteille M. Nuclear bodies as proteinaceous structures containing ribonucleoproteins. J Ultrastruct Res. 1972 Jan;38(1):174–187. doi: 10.1016/s0022-5320(72)90091-3. [DOI] [PubMed] [Google Scholar]
- GRANBOULAN N., TOURNIER P., WICKER R., BERNHARD W. An electron microscope study of the development of SV40 virus. J Cell Biol. 1963 May;17:423–441. doi: 10.1083/jcb.17.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I., Morel-Maroger L., Châtelet F., Maury C., Tovey M., Bandu M. T., Buywid J., Delauche M. Delay in growth and the development of nephritis in rats treated with interferon preparations in the neonatal period. Am J Pathol. 1979 May;95(2):329–346. [PMC free article] [PubMed] [Google Scholar]
- Grimley P. M., Barry D. W., Schaff Z. Induction of tubular structures in the endoplasmic reticulum of human lymphoid cells by treatment with 5-bromo-2'-deoxyuridine. J Natl Cancer Inst. 1973 Dec;51(6):1751–1760. doi: 10.1093/jnci/51.6.1751. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y., Koprowski H., Müller D., ter Meulen V., Käckell Y. M. Morphogenesis and structure of a virus in cells cultured from brain tissue from two cases of multiple sclerosis. Lab Invest. 1973 Apr;28(4):494–500. [PubMed] [Google Scholar]
- Maiztegui J. I., Laguens R. P., Cossio P. M., Casanova M. B., de la Vega M. T., Ritacco V., Segal A., Fernández N. J., Arana R. M. Ultrastructural and immunohistochemical studies in five cases of Argentine hemorrhagic fever. J Infect Dis. 1975 Jul;132(1):35–53. doi: 10.1093/infdis/132.1.35. [DOI] [PubMed] [Google Scholar]
- Margolis G., Kilham L., Baringer J. R. Identity of cowdry type B inclusions and nuclear bodies: observations in reovirus encephalitis. Exp Mol Pathol. 1975 Oct;23(2):228–244. doi: 10.1016/0014-4800(75)90021-0. [DOI] [PubMed] [Google Scholar]
- Martinez A. J., Oya T., Jabbour J. T., Dueñas D. Subacute sclerosing panencephalitis (SSPE). Reappraisal of nuclear, cytoplasmic and axonal inclusions ultrastructural study of eight cases. Acta Neuropathol. 1974 May 31;28(1):1–13. doi: 10.1007/BF00687513. [DOI] [PubMed] [Google Scholar]
- Payne C. M., Sibley W. A. Intranuclear inclusions in a case of Creutzfeldt-Jakob disease: an ultrastructural study. Acta Neuropathol. 1975;31(4):353–361. doi: 10.1007/BF00687930. [DOI] [PubMed] [Google Scholar]
- Perez G. O., Bjornsson S., Ross A. H., Aamato J., Rothfield N. A mini-epidemic of Goodpasture's syndrome clinical and immunological studies. Nephron. 1974;13(2):161–173. doi: 10.1159/000180389. [DOI] [PubMed] [Google Scholar]
- Phillips P. E., Christian C. L. Virus antibodies in systemic lupus erythematosus and other connective tissue diseases. Ann Rheum Dis. 1973 Sep;32(5):450–456. doi: 10.1136/ard.32.5.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesing T. G., Landau B. J., Crowell R. L. Limited persistence of viral antigen in coxsackievirus B3 induced heart disease in mice. Proc Soc Exp Biol Med. 1979 Mar;160(3):382–386. doi: 10.3181/00379727-160-40455. [DOI] [PubMed] [Google Scholar]
- Runeberg L., Collan Y., Jokinen E. J., Lähdevirta J., Aro A. Hypomagnesemia due to renal disease of unknown etiology. Am J Med. 1975 Dec;59(6):873–881. doi: 10.1016/0002-9343(75)90481-7. [DOI] [PubMed] [Google Scholar]
- Schaff Z., Barry D. W., Grimley P. M. Cytochemistry of tubuloreticular structures in lymphocytes from patients with systemic lupus erythematosus and in cultured human lymphoid cells: comparison to a paramyxovirus. Lab Invest. 1973 Dec;29(6):577–586. [PubMed] [Google Scholar]
- Talal N. Immunologic and viral factors in the pathogenesis of systemic lupus erythematosus. Arthritis Rheum. 1970 Nov-Dec;13(6):887–894. doi: 10.1002/art.1780130620. [DOI] [PubMed] [Google Scholar]
- Ulrich J., Kidd M. Subacute inclusion body encephalitis. A histological and electron microscopical study. Acta Neuropathol. 1966 Jul 7;6(4):359–370. doi: 10.1007/BF00688164. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Smith R. C. Goodpasture's syndrome associated with influenza A2 virus infection. Ann Intern Med. 1972 Jan;76(1):91–94. doi: 10.7326/0003-4819-76-1-91. [DOI] [PubMed] [Google Scholar]
- de THE, RIVIERE M., BERNHARD W. [Examination by electron microscope of the VX2 tumor of the domestic rabbit derived from the Shope papilloma]. Bull Assoc Fr Etud Cancer. 1960 Oct-Dec;47:570–584. [PubMed] [Google Scholar]