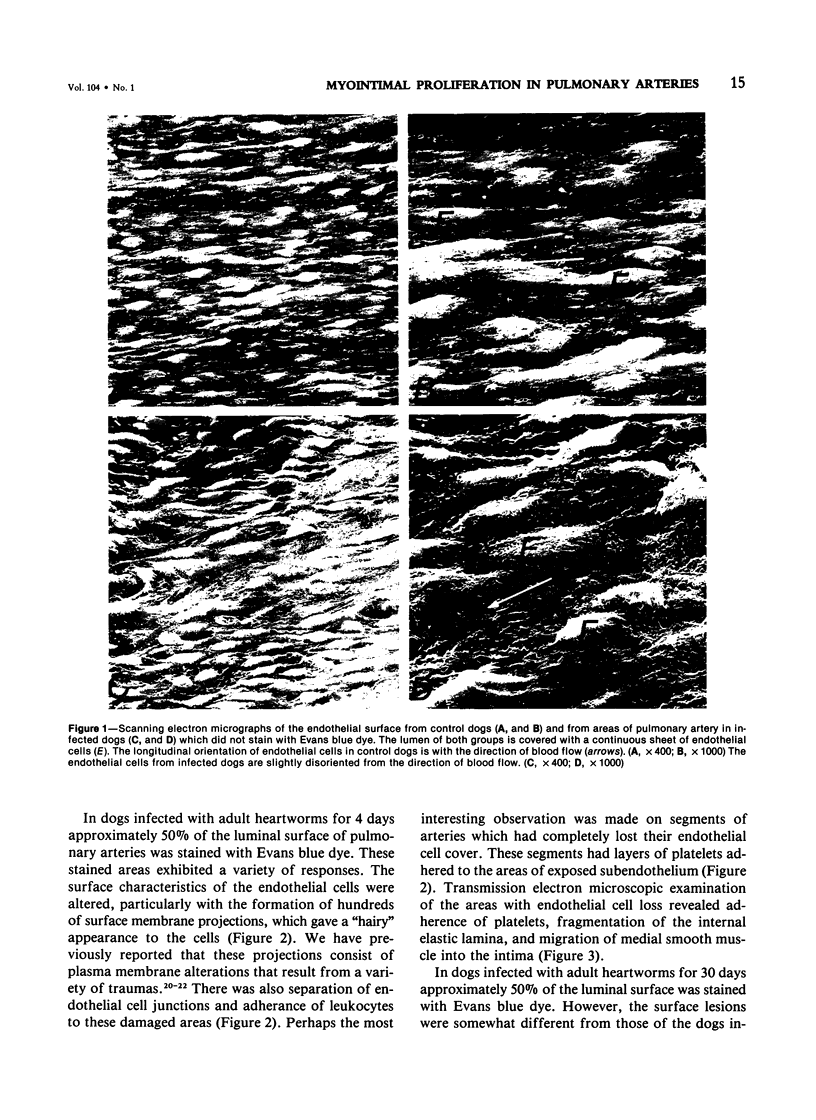
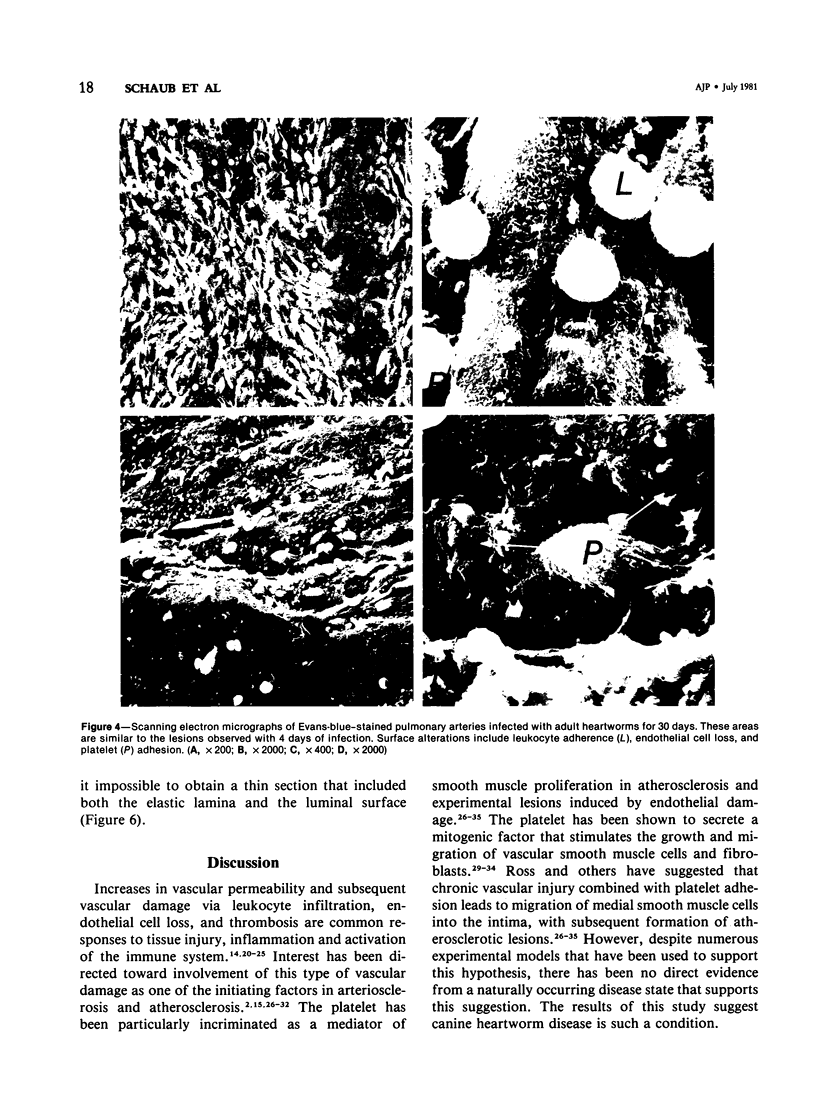
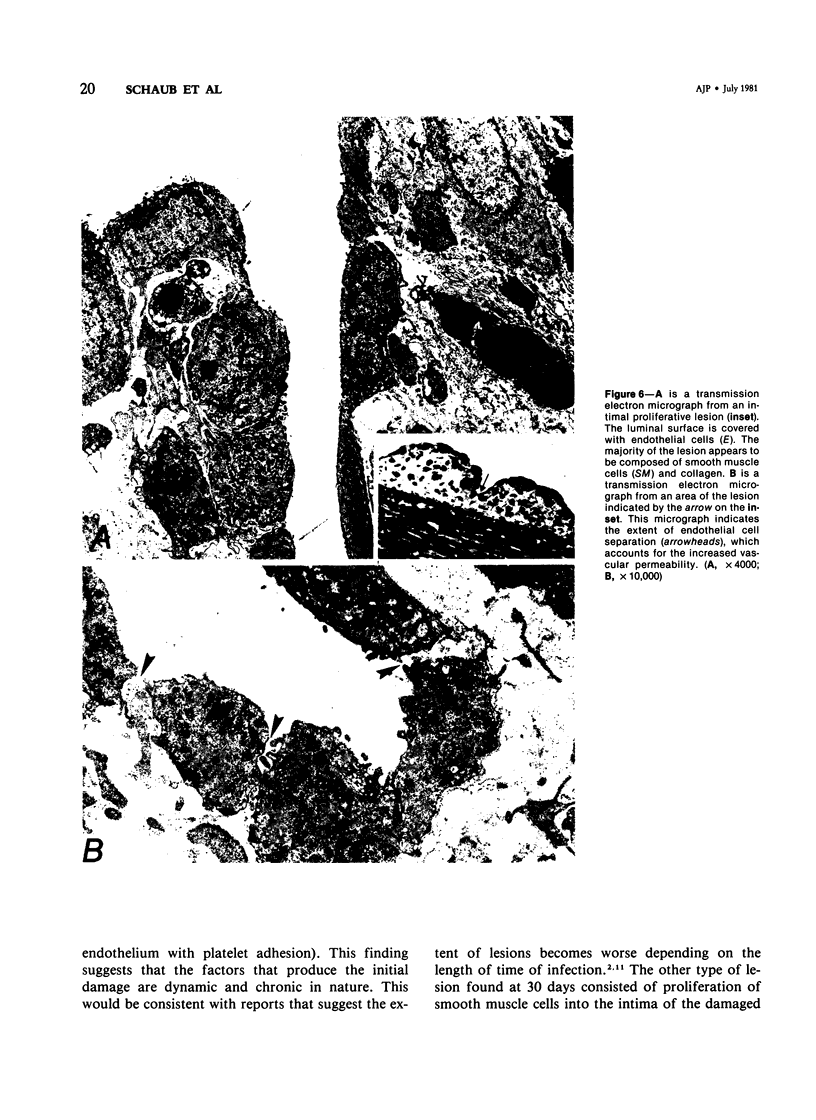
Abstract
Pulmonary arteries were studied by scanning and transmission electron microscopy in 15 preconditioned dogs. Five dogs were control animals, while 10 dogs were studied 4 and 30 days following transplantation of adult heartworms into the pulmonary arteries. Evan's blue dye was used to locate areas of vascular damage. Pulmonary arteries from control dogs exhibited no Evan's blue staining. The surface and ultrastructural characteristics of these blood vessels were comparable to normal peripheral blood vessels. Pulmonary arteries removed from dogs after 4 days of heartworm infection exhibited extensive staining with Evan's blue. These stained areas had disrupted endothelium with many platelets adhered to the exposed subendothelium. In addition, leukocytes were attached to adjacent areas of damaged endothelium. Pulmonary arteries of dogs infected with heartworms for 30 days also exhibited extensive staining with Evan's blue. The blue-stained areas in this group had two typical responses. On some portions the lesions were similar to those seen at 4 days (ie, loss of endothelium with platelet and leukocyte adhesion), while other stained areas had complex lesions that projected from the surface into the lumen of the blood vessel. These lesions were endothelialized, and transmission electron microscopy revealed that they consisted of large numbers of smooth muscle cells that had migrated through the internal elastic lamina. The findings in the 30-day infection group suggest that the proliferative lesion formation was a result of an ongoing active process of endothelial loss and plateletleukocyte adhesion. The characteristic response of canine pulmonary arteries to the presence of heartworms (endothelial loss, platelet-leukocyte adhesion, and development of myoproliferative intimal lesions) suggests that this condition is a potential model for study of the early vascular changes that produce myointimal proliferation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADCOCK J. L. Pulmonary arterial lesions in canine dirofilariasis. Am J Vet Res. 1961 Jul;22:655–662. [PubMed] [Google Scholar]
- Bell F. P., Somer J. B., Craig I. H., Schwartz C. J. Patterns of aortic Evans blue uptake in vivo and in vitro. Atherosclerosis. 1972 Nov-Dec;16(3):369–375. doi: 10.1016/0021-9150(72)90084-6. [DOI] [PubMed] [Google Scholar]
- Cohen I. Role of endothelial injury and platelets in atherogenesis. Artery. 1979 Mar;5(3):237–245. [PubMed] [Google Scholar]
- Fishman J. A., Ryan G. B., Karnovsky M. J. Endothelial regeneration in the rat carotid artery and the significance of endothelial denudation in the pathogenesis of myointimal thickening. Lab Invest. 1975 Mar;32(3):339–351. [PubMed] [Google Scholar]
- Friedman R. J., Stemerman M. B., Wenz B., Moore S., Gauldie J., Gent M., Tiell M. L., Spaet H. The effect of thrombocytopenia on experimental arteriosclerotic lesion formation in rabbits. Smooth muscle cell proliferation and re-endothelialization. J Clin Invest. 1977 Nov;60(5):1191–1201. doi: 10.1172/JCI108872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg I. D., Stemerman M. B., Handin R. I. Vascular permeation of platelet factor 4 after endothelial injury. Science. 1980 Aug 1;209(4456):611–612. doi: 10.1126/science.6994228. [DOI] [PubMed] [Google Scholar]
- Guyton J. R., Karnovsky M. J. Smooth muscle cell proliferation in the occluded rat carotid artery: lack of requirement for luminal platelets. Am J Pathol. 1979 Mar;94(3):585–602. [PMC free article] [PubMed] [Google Scholar]
- HENNIGAR G. R., FERGUSON R. W. Pulmonary vascular sclerosis as a result of Dirofilaria immitis infection in dogs. J Am Vet Med Assoc. 1957 Oct 1;131(7):336–340. [PubMed] [Google Scholar]
- JACKSON R. F., VON LICHTENBERG F., OTTO G. F. Occurrence of adult heartworms in the venae cavae of dogs. J Am Vet Med Assoc. 1962 Jul 1;141:117–121. [PubMed] [Google Scholar]
- Jorgensen L., Packham M. A., Rowsell H. C., Mustard J. F. Deposition of formed elements of blood on the intima and signs of intimal injury in the aorta of rabbit, pig, and man. Lab Invest. 1972 Sep;27(3):341–350. [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Minick C. R. Immunologic arterial injury in atherogenesis. Ann N Y Acad Sci. 1976;275:210–227. doi: 10.1111/j.1749-6632.1976.tb43355.x. [DOI] [PubMed] [Google Scholar]
- Munnell J. F., Weldon J. S., Lewis R. E., Thrall D. E., McCall J. W. Intimal lesions of the pulmonary artery in dogs with experimental dirofilariasis. Am J Vet Res. 1980 Jul;41(7):1108–1112. [PubMed] [Google Scholar]
- Mustard J. F., Packham M. A. The role of blood and platelets in atherosclerosis and the complications of atherosclerosis. Thromb Diath Haemorrh. 1975 Jun 30;33(3):444–456. [PubMed] [Google Scholar]
- Packham M. A., Rowsell H. C., Jorgensen L., Mustard J. F. Localized protein accumulation in the wall of the aorta. Exp Mol Pathol. 1967 Oct;7(2):214–232. doi: 10.1016/0014-4800(67)90031-7. [DOI] [PubMed] [Google Scholar]
- Ratliff N. B., Gerrard J. M., White J. G. Platelet-leukocyte interactions following arterial endothelial injury. Am J Pathol. 1979 Aug;96(2):567–580. [PMC free article] [PubMed] [Google Scholar]
- Rawlings C. A. Acute response of pulmonary blood flow and right ventricular function to Dirofilaria immitis adults and microfilaria. Am J Vet Res. 1980 Feb;41(2):244–249. [PubMed] [Google Scholar]
- Rawlings C. A. Cardiopulmonary function in the dog with Dirofilaria immitis infection: during infection and after treatment. Am J Vet Res. 1980 Mar;41(3):319–325. [PubMed] [Google Scholar]
- Rawlings C. A. Pulmonary vascular response of dogs with heartworm disease. Can J Comp Med. 1978 Oct;42(4):452–459. [PMC free article] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976 Aug 19;295(8):420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Harker L. Response to injury and atherogenesis. Am J Pathol. 1977 Mar;86(3):675–684. [PMC free article] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Harker L. Hyperlipidemia and atherosclerosis. Science. 1976 Sep 17;193(4258):1094–1100. doi: 10.1126/science.822515. [DOI] [PubMed] [Google Scholar]
- Rutherford R. B., Ross R. Platelet factors stimulate fibroblasts and smooth muscle cells quiescent in plasma serum to proliferate. J Cell Biol. 1976 Apr;69(1):196–203. doi: 10.1083/jcb.69.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G. B., Majno G. Acute inflammation. A review. Am J Pathol. 1977 Jan;86(1):183–276. [PMC free article] [PubMed] [Google Scholar]
- Schafer A. I., Handin R. I. The role of platelets in thrombotic and vascular disease. Prog Cardiovasc Dis. 1979 Jul-Aug;22(1):31–52. doi: 10.1016/0033-0620(79)90002-1. [DOI] [PubMed] [Google Scholar]
- Schaub R. G., Lynch P. R., Stewart G. J. The response of canine veins to three types of abdominal surgery: a scanning and transmission electron microscopic study. Surgery. 1978 Apr;83(4):411–424. [PubMed] [Google Scholar]
- Schaub R. G., Rawlings C. A. Pulmonary vascular response during phases of canine heartworm disease: scanning electron microscopic study. Am J Vet Res. 1980 Jul;41(7):1082–1089. [PubMed] [Google Scholar]
- Schaub R. G., Rawlings C. A., Stewart G. J. Scanning electron microscopy of canine pulmonary arteries and veins. Am J Vet Res. 1980 Sep;41(9):1441–1446. [PubMed] [Google Scholar]
- Stehbens W. E. Endothelial permeability in experimental aneurysms and arteriovenous fistulas in rabbits as demonstrated by the uptake of Evans blue. Atherosclerosis. 1978 Aug;30(4):343–349. doi: 10.1016/0021-9150(78)90127-2. [DOI] [PubMed] [Google Scholar]
- Stewart G. J., Ritchie W. G., Lynch P. R. Venous endothelial damage produced by massive sticking and emigration of leukocytes. Am J Pathol. 1974 Mar;74(3):507–532. [PMC free article] [PubMed] [Google Scholar]
- Stewart G. J., Stern H. R., Schaub R. G. Endothelial alterations, deposition of blood elements and increased accumulation of 131I-albumin in canine jugular veins following abdominal surgery. Thromb Res. 1978 Mar;12(3):555–563. doi: 10.1016/0049-3848(78)90326-2. [DOI] [PubMed] [Google Scholar]
- VON LICHTENBERG F., JACKSON R. F., OTTO G. F. Hepatic lesions in dogs with dirofilariasis. J Am Vet Med Assoc. 1962 Jul 1;141:121–128. [PubMed] [Google Scholar]