Abstract
To investigate the neutrophil-neutrophil interactions of the newborn for possible clues to the etiology of decreased newborn neutrophil (PMN) chemotaxis, the authors compared adult and newborn C5a-induced PMN aggregation and chemotaxis at various PMN concentrations. Using Craddock's technique of C5a-induced aggregation, the authors found that the newborn lacks the normal biphasic aggregation-deaggregation seen in the adult, suggesting irreversible aggregation similar to that seen when adult PMNs are pretreated with cytochalasin-B. Chemotaxis of adult and newborn PMNs was studied with a modified Gallin radiolabel technique. A linear correlation between PMN concentration and corrected chemotactic response was found with both adult (r2 = 0.93) and newborn (r2 = 0.90) PMNs in the range 0.1 X 10(6) to 20 X 10(6) PMNs/ml. Random migration was not augmented by increased PMN concentration. The augmentation of newborn PMN chemotaxis was less than that of the adult (adult slope = 2426; newborn slope = 983). Irreversible newborn PMN aggregation may be the underlying event producing decreased PMN chemotaxis and interfering with the normal chemotactic augmentation caused by increased PMN concentration.
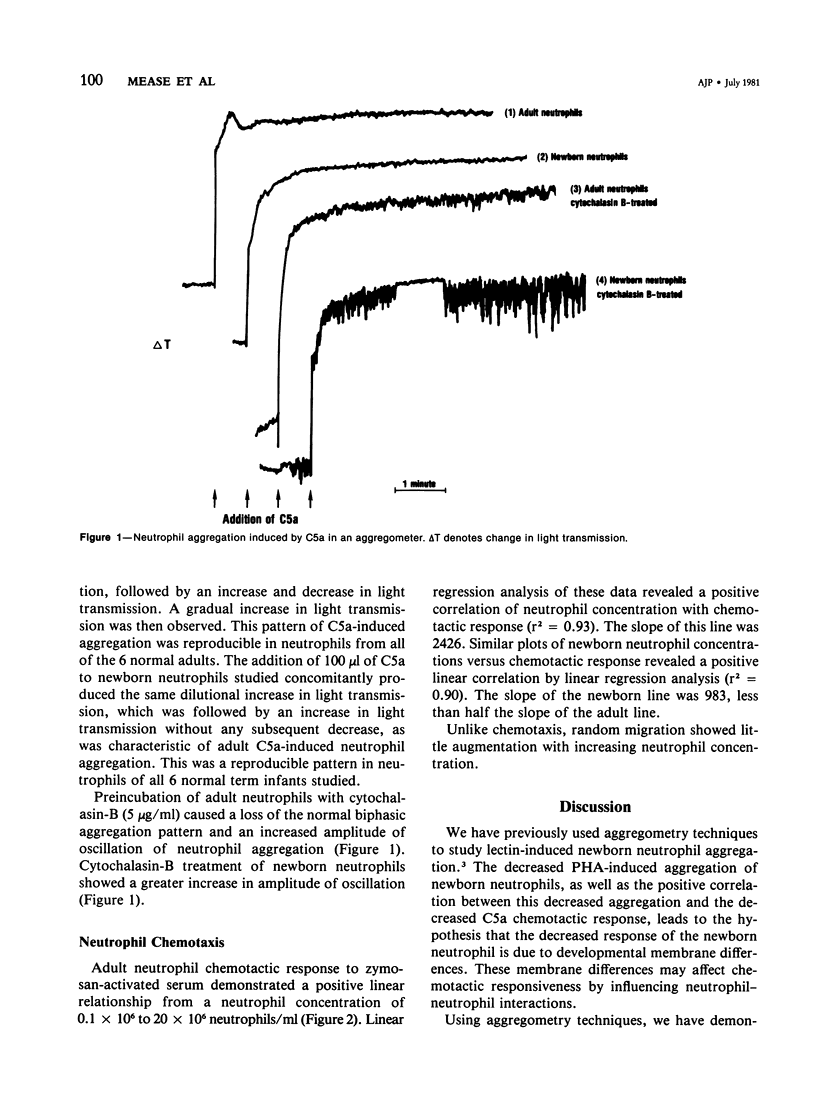
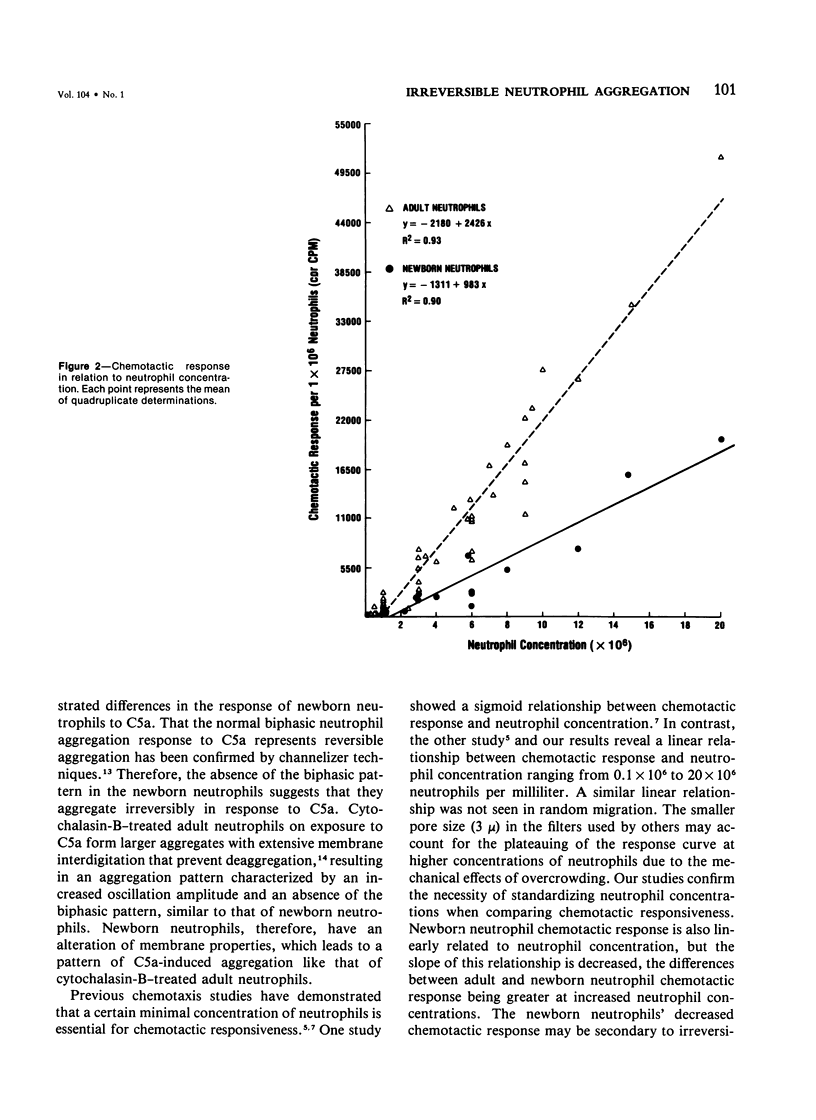
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Christensen R. D., Rothstein G. Exhaustion of mature marrow neutrophils in neonates with sepsis. J Pediatr. 1980 Feb;96(2):316–318. doi: 10.1016/s0022-3476(80)80837-7. [DOI] [PubMed] [Google Scholar]
- Craddock P. R., Hammerschmidt D. E., Moldow C. F., Yamada O., Jacob H. S. Granulocyte aggregation as a manifestation of membrane interactions with complement: possible role in leukocyte margination, microvascular occlusion, and endothelial damage. Semin Hematol. 1979 Apr;16(2):140–147. [PubMed] [Google Scholar]
- Craddock P. R., Hammerschmidt D., White J. G., Dalmosso A. P., Jacob H. S. Complement (C5-a)-induced granulocyte aggregation in vitro. A possible mechanism of complement-mediated leukostasis and leukopenia. J Clin Invest. 1977 Jul;60(1):260–264. doi: 10.1172/JCI108763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock P. R., White J. G., Jacob H. S. Potentiation of complement (C5a)-induced granulocyte aggregation by cytochalasin B. J Lab Clin Med. 1978 Mar;91(3):490–499. [PubMed] [Google Scholar]
- Gallin J. I., Clark R. A., Kimball H. R. Granulocyte chemotaxis: an improved in vitro assay employing 51 Cr-labeled granulocytes. J Immunol. 1973 Jan;110(1):233–240. [PubMed] [Google Scholar]
- Gallin J. I., Wright D. G., Schiffmann E. Role of secretory events in modulating human neutrophil chemotaxis. J Clin Invest. 1978 Dec;62(6):1364–1374. doi: 10.1172/JCI109257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook W. A., Siraganian R. P., Wahl S. M. Complement-induced histamine release from human basophils. I. Generation of activity in human serum. J Immunol. 1975 Apr;114(4):1185–1190. [PubMed] [Google Scholar]
- Jacob H. S., Craddock P. R., Hammerschmidt D. E., Moldow C. F. Complement-induced granulocyte aggregation: an unsuspected mechanism of disease. N Engl J Med. 1980 Apr 3;302(14):789–794. doi: 10.1056/NEJM198004033021407. [DOI] [PubMed] [Google Scholar]
- Mease A. D., Fischer G. W., Hunter K. W., Ruymann F. B. Decreased phytohemagglutinin-induced aggregation and C5a-induced chemotaxis of human newborn neutrophils. Pediatr Res. 1980 Feb;14(2):142–146. doi: 10.1203/00006450-198002000-00015. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Ward P. A. Neutrophil aggregation and swelling induced by chemotactic agents. J Immunol. 1977 Jul;119(1):232–239. [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. J., Becker E. L., Ward P. A. Substances which aggregate neutrophils. Mechanism of action. Am J Pathol. 1978 Jul;92(1):155–166. [PMC free article] [PubMed] [Google Scholar]
- Patten E., Gallin J. I., Clark R. A., Kimball H. R. Effects of cell concentration and various anticoagulants on neutrophil migration. Blood. 1973 May;41(5):711–719. [PubMed] [Google Scholar]
- Takeuchi A., Persellin R. H. Cellular augmentation of polymorphonuclear leukocyte chemotaxis. Am J Physiol. 1979 Jan;236(1):C22–C29. doi: 10.1152/ajpcell.1979.236.1.C22. [DOI] [PubMed] [Google Scholar]
- Vallota E. H., Müller-Eberhard H. J. Formation of C3a and C5a anaphylatoxins in whole human serum after inhibition of the anaphylatoxin inactivator. J Exp Med. 1973 May 1;137(5):1109–1123. doi: 10.1084/jem.137.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]


