Abstract
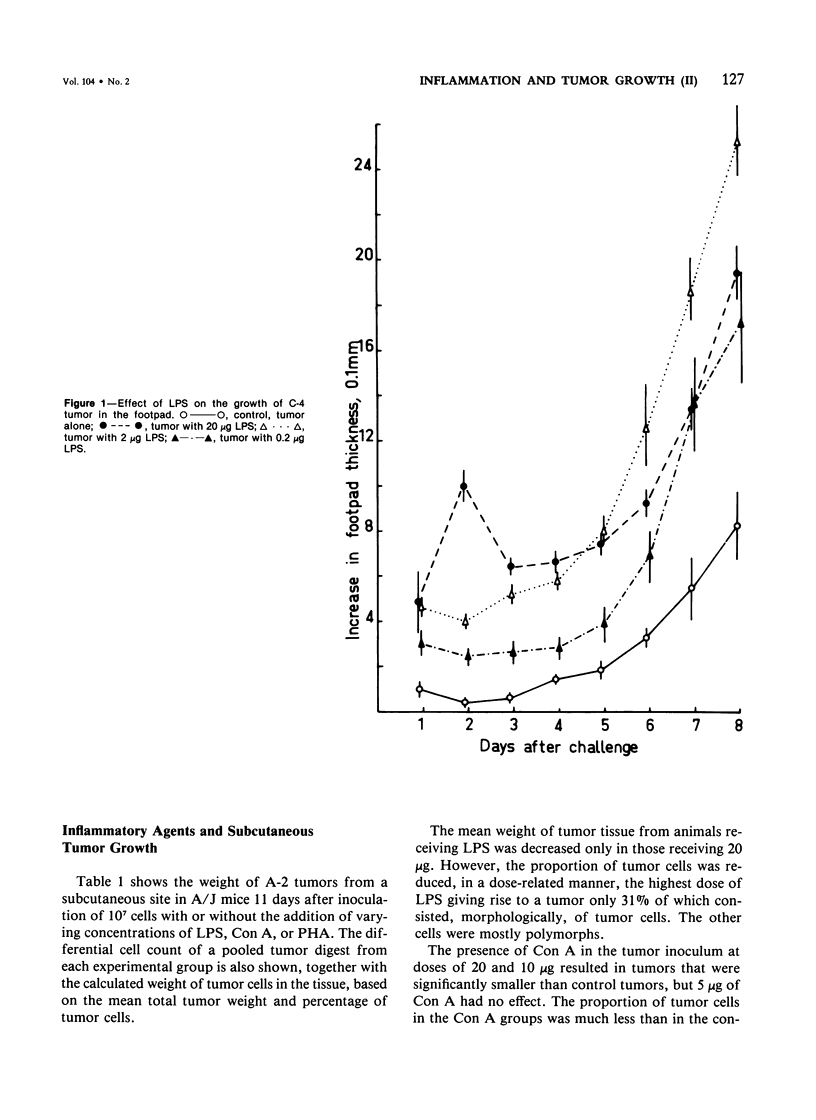
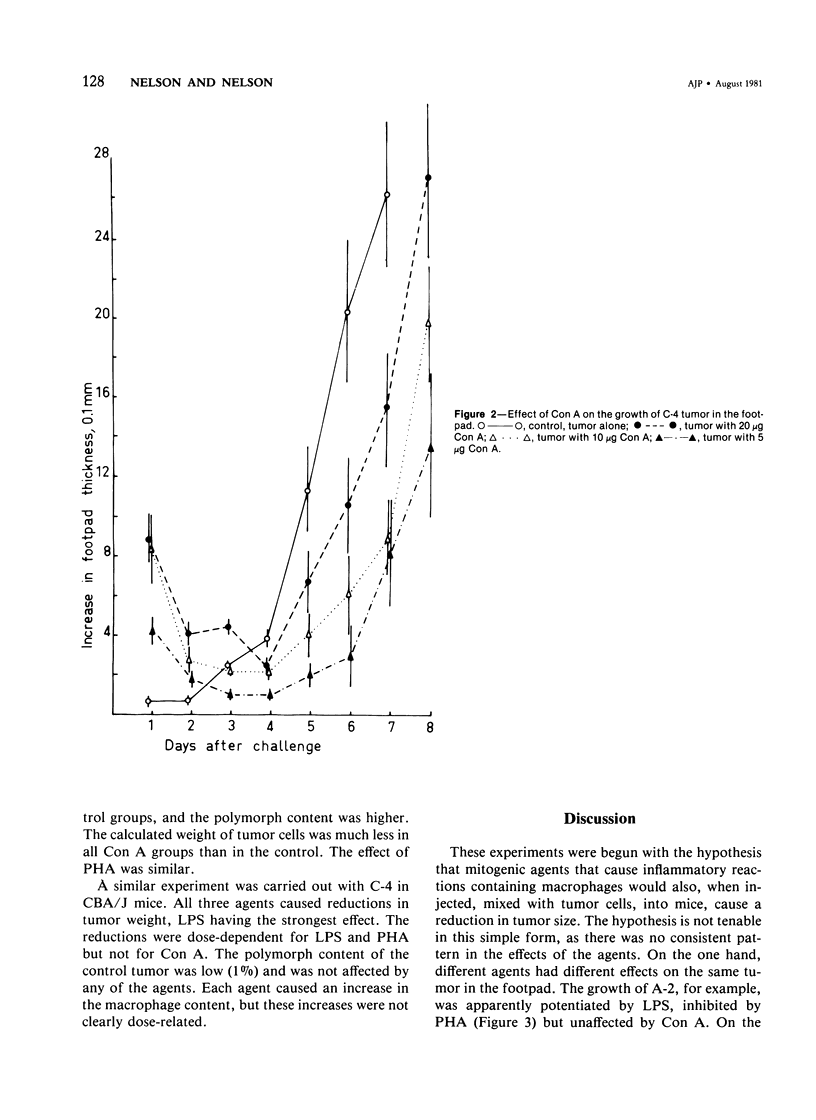
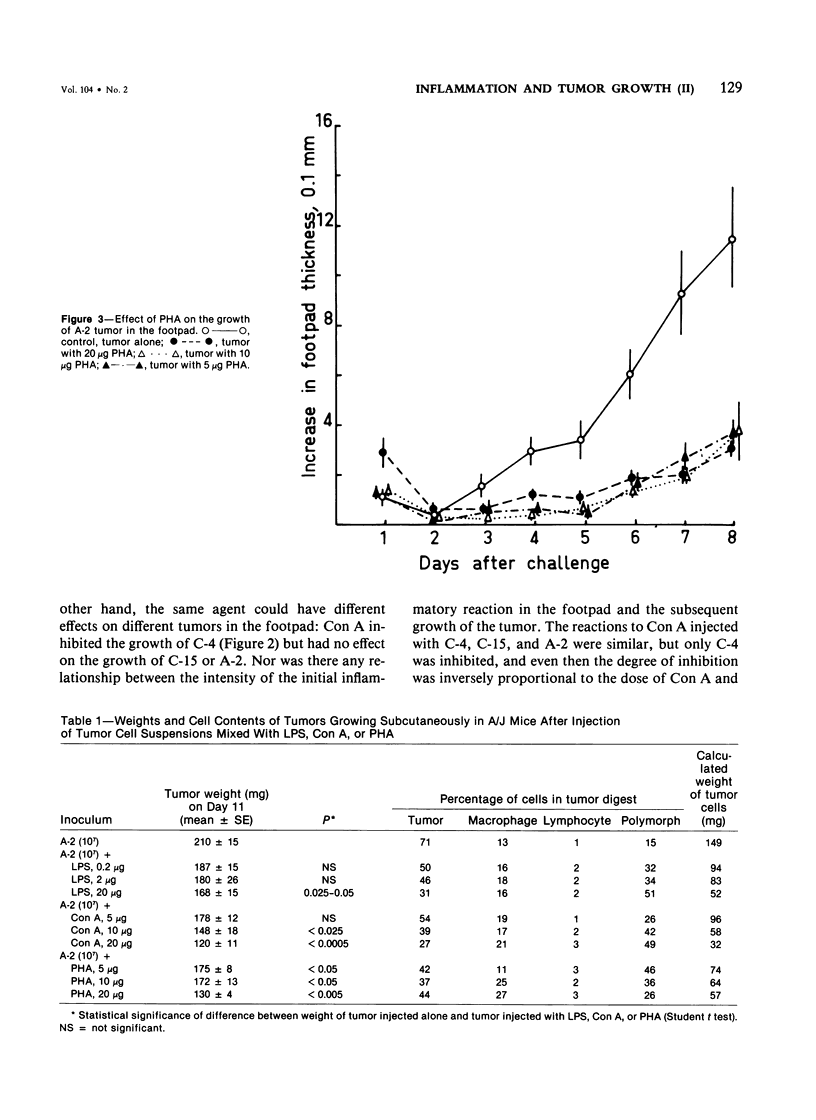
Experiments were carried out to determine whether the growth of tumors could be influenced by local inflammatory reactions induced by mitogens; Gram-negative bacterial lipopolysaccharide (LPS), concanavalin A (Con A) and phytohemagglutinin (PHA). Mice received injections, beneath the footpad or subcutaneously in the flank, of cells of syngeneic chemically induced fibrosarcomas with or without varying doses of mitogen. In the footpad (a) LPS caused a dose-dependent increase in the size; (b) Con A caused a decrease in the size of one of the three tumors, the decrease being inversely related to the dose of Con A; (c) PHA caused a dose-dependent decrease in the size of all three tumors: (d) PHA caused much smaller macroscopic inflammatory reactions than LPS or Con A. Subcutaneously injected tumor growth was inhibited by all three agents. Subcutaneous tumors contained a higher proportion of host inflammatory cells when mitogens had been mixed with the tumor inoculum. It is concluded that mitogens that can induce inflammatory reactions in mice can also bring about some suppression of tumor growth but that the depression is site-dependent and not clearly related to the apparent intensity of inflammation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berendt M. J., Saluk P. H. Tumor inhibition in mice by lipopolysaccharide-induced peritoneal cells and an induced soluble factor. Infect Immun. 1976 Oct;14(4):965–969. doi: 10.1128/iai.14.4.965-969.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraschi D., Meltzer M. S. Defective tumoricidal capacity of macrophages from A/J mice. I. Characterization of the macrophage cytotoxic defect after in vivo and in vitro activation stimuli. J Immunol. 1979 Apr;122(4):1587–1591. [PubMed] [Google Scholar]
- Doe W. F., Henson P. M. Macrophage stimulation by bacterial lipopolysaccharides. I. Cytolytic effect on tumor target cells. J Exp Med. 1978 Aug 1;148(2):544–556. doi: 10.1084/jem.148.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gately M. K., Mayer M. M. Purification and characterization of lymphokines: an approach to the study of molecular mechanisms of cell-mediated immunity. Prog Allergy. 1978;25:106–162. [PubMed] [Google Scholar]
- Gershon R. K., Askenase P. W., Gershon M. D. Requirement for vasoactive amines for production of delayed-type hypersensitvity skin reactions. J Exp Med. 1975 Sep 1;142(3):732–747. doi: 10.1084/jem.142.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna M. G., Jr, Zbar B., Rapp H. J. Histopathology of tumor regression after intralesional injection of Mycobacterium bovis., 2. Comparative effects of vaccinia virus, oxazolone, and turpentine. J Natl Cancer Inst. 1972 Jun;48(6):1697–1707. [PubMed] [Google Scholar]
- Hay J. B., Hobbs B. B., Johnston M. G., Movat H. Z. The role of hyperemia in cellular hypersensitivity reactions. Int Arch Allergy Appl Immunol. 1977;55(1-6):324–331. doi: 10.1159/000231943. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Chapman H. A., Jr, Weinberg J. B. The macrophage as an antineoplastic surveillance cell: biological perspectives. J Reticuloendothel Soc. 1978 Nov;24(5):549–570. [PubMed] [Google Scholar]
- Hopper K. E., Harrison J., Nelson D. S. Partial characterization of anti-tumor effector macrophages in the peritoneal cavities of concomitantly immune mice and mice injected with macrophage-stimulating agents. J Reticuloendothel Soc. 1979 Sep;26(3):259–271. [PubMed] [Google Scholar]
- Kearney R., Nelson D. S. Concomitant immunity to syngeneic methylcholanthrene-induced tumours in mice. Occurrence and specificity of concomitant immunity. Aust J Exp Biol Med Sci. 1973 Dec;51(6):723–735. doi: 10.1038/icb.1973.70. [DOI] [PubMed] [Google Scholar]
- Lagrange P. H., Thickstun P. M. In vivo antitumor activity of various forms of delayed-type hypersensitivity in mice. J Natl Cancer Inst. 1979 Feb;62(2):429–436. [PubMed] [Google Scholar]
- Levy M. H., Wheelock E. F. The role of macrophages in defense against neoplastic disease. Adv Cancer Res. 1974;20:131–163. doi: 10.1016/s0065-230x(08)60110-4. [DOI] [PubMed] [Google Scholar]
- Lewis A. J., Cottney J., Nelson D. J. Mechanisms of phytohaemagglutinin-P-, concanavalin-A- and kaolin-induced oedemas in the rat. Eur J Pharmacol. 1976 Nov;40(1):1–8. doi: 10.1016/0014-2999(76)90347-2. [DOI] [PubMed] [Google Scholar]
- More D. G., Penrose J. M., Kearney R., Nelson D. S. Immunological induction of DNA synthesis in mouse peritoneal macrophages. An expression of cell-mediated-immunity. Int Arch Allergy Appl Immunol. 1973;44(5):611–630. doi: 10.1159/000230965. [DOI] [PubMed] [Google Scholar]
- Nelson M., Nelson D. S., Hopper K. E. I. Tumor growth in mice with depressed capacity to mount inflammatory responses: possible role of macrophages. Am J Pathol. 1981 Aug;104(2):114–124. [PMC free article] [PubMed] [Google Scholar]
- Nelson M., Nelson D. S. Macrophages and resistance to tumours. I. Inhibition of delayed-type hypersensitivity reactions by tumour cells and by soluble prducts affecting macrophages. Immunology. 1978 Feb;34(2):277–290. [PMC free article] [PubMed] [Google Scholar]
- Nelson M., Nelson D. S. Macrophages and resistance to tumours: influence of agents affecting macrophages and delayed-type hypersensitivity on resistance to tumours inducing concomitant immunity. Aust J Exp Biol Med Sci. 1978 Apr;56(2):211–223. [PubMed] [Google Scholar]
- Palladino M. A., Thorbecke G. J. Immunity to transplantable carcinogen-induced fibrosarcomas in B2/B2 chickens. III. Tumor growth inhibition by local delayed hypersensitivity reactions to unrelated antigens. Eur J Immunol. 1978 Apr;8(4):257–262. doi: 10.1002/eji.1830080408. [DOI] [PubMed] [Google Scholar]
- Paranjpe M. S., Boone C. W. Stimulated growth of syngeneic tumors at the site of an ongoing delayed-hypersensitivity reaction to tuberculin in BALB-c mice. J Natl Cancer Inst. 1974 Apr;52(4):1297–1299. doi: 10.1093/jnci/52.4.1297. [DOI] [PubMed] [Google Scholar]
- STETSON C. A., Jr Studies on the mechanism of the Shwartzman phenomenon; similarities between reactions to endotoxins and certain reactions of bacterial allergy. J Exp Med. 1955 Apr 1;101(4):421–436. doi: 10.1084/jem.101.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinebring W. R., Stevens D. Escherichia coli endotoxin effect on a methylcholanthrene-induced sarcoma in the hamster cheekpouch. Proc Soc Exp Biol Med. 1977 Nov;156(2):229–235. doi: 10.3181/00379727-156-39912. [DOI] [PubMed] [Google Scholar]
- Weinberg J. B., Chapman H. A., Jr, Hibbs J. B., Jr Characterization of the effects of endotoxin on macrophage tumor cell killing. J Immunol. 1978 Jul;121(1):72–80. [PubMed] [Google Scholar]
- Wells J. H., Cain W. A., Wells R. S., Bozalis J. R. Suppression of tuberculin and phytohemagglutinin skin tests by large tumors in inbred mice. Int Arch Allergy Appl Immunol. 1974;47(3):362–368. doi: 10.1159/000231229. [DOI] [PubMed] [Google Scholar]
- Yarkoni E., Ruco L. P., Rapp H. J., Meltzer M. S. Histopathology of tumor regression by cord factor, turpentine or endotoxin, dissociation of therapy and granuloma formation. Eur J Cancer. 1979 Nov;15(11):1401–1407. doi: 10.1016/0014-2964(79)90118-x. [DOI] [PubMed] [Google Scholar]


