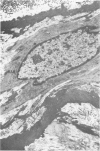
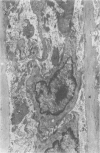
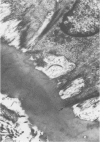
Abstract
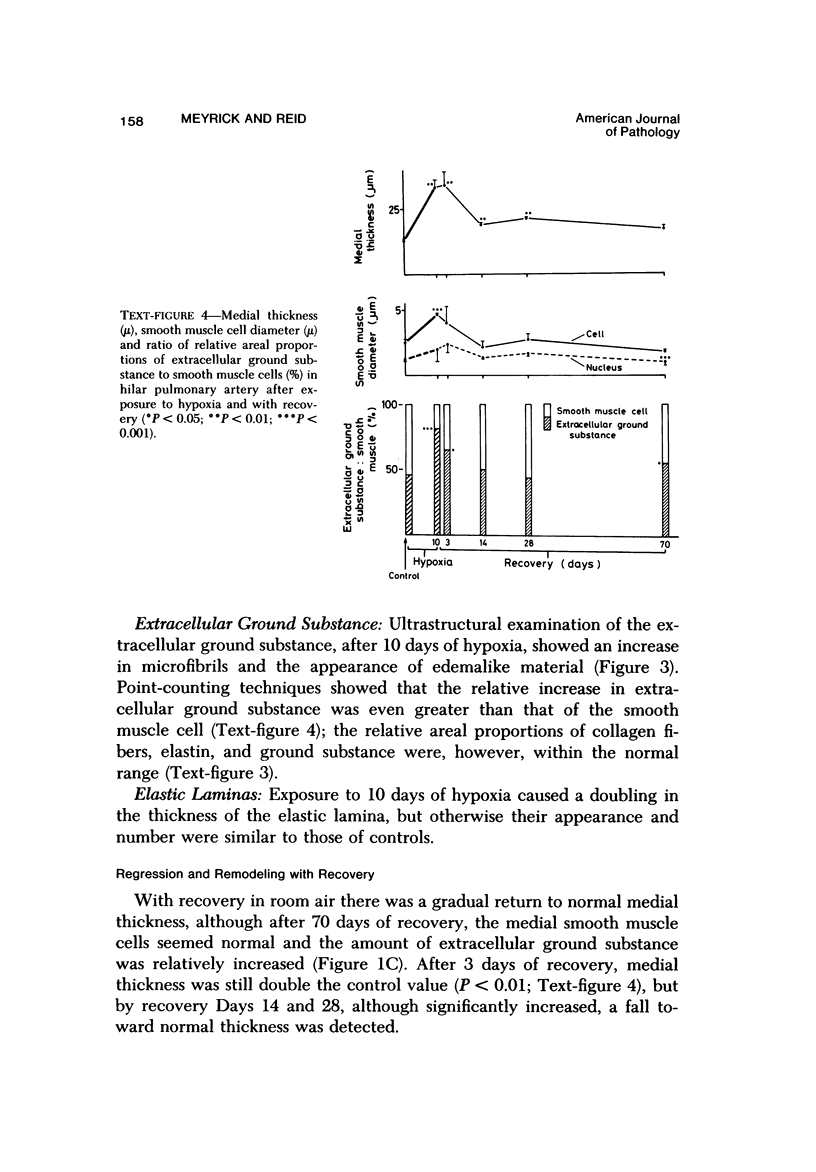
Chronic hypobaric hypoxia induces structural features characteristic of pulmonary hypertension, but little is known of their reversal. In the present study, rats have been exposed to hypobaric hypoxia for 10 days and subsequently allowed to recover in room air for 3, 14, 28, or 70 days. With the use of 1-mu sections and electron-microscopic and point-counting techniques, the regression of the medial and adventitial changes in the rat hilar intrapulmonary artery has been followed. In the media, 10-day hypoxia causes more than a doubling in thickness due to 1) hypertrophy of smooth muscle cells, particularly of rough sarcoplasmic reticulum and Golgi apparatus; 2) an increase in extracellular connective tissue, microfibrils, collagen fibers, and elastin, and 3) edemalike fluid. In addition, the elestic laminas are doubled in thickness, and myofilamentous processes of the hypertrophied smooth muscle cells contact them. After just 3 days' recovery, some cells have already returned to normal diameter, although medial thickening is unchanged. By Day 14 and at Days 28 and 70 of recovery, medial thickness and cell diameter are within the normal range, and by recovery Day 70, there is a significant increase in the relative areal proportion of extracellular collagen fibers and a decrease in elastin (P less than 0.001). Hypoxia also produces a more than twofold increase in adventitial thickness. Hypertrophy of fibroblasts and an increase in their number contribute to the thickening, as does as increase in collagen fibers. During 3-70 days of recovery, thickness is gradually reduced to normal levels, although it is still significantly above normal at Days 3, 14, and 28. The increases in thickness at these times are due mainly to the accumulation of collagen fibers, which are still apparent after 70 days of recovery. Thus, hypoxia causes a doubling in thickness of the medial and adventitial coats of the hilar muscular pulmonary artery, which with recovery regain near normal thickness but whose structure is altered. The increase in collagen fibers contributes to contracture and reduced distensibility in these vessels, which is apparent in arteriograms as narrowed lumen diameter.
Full text
PDF

























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham A. S., Kay J. M., Cole R. B., Pincock A. C. Haemodynamic and pathological study of the effect of chronic hypoxia and subsequent recovery of the heart and pulmonary vasculature of the rat. Cardiovasc Res. 1971 Jan;5(1):95–102. doi: 10.1093/cvr/5.1.95. [DOI] [PubMed] [Google Scholar]
- Burke J. M., Ross R. Collagen synthesis by monkey arterial smooth muscle cells during proliferation and quiescence in culture. Exp Cell Res. 1977 Jul;107(2):387–395. doi: 10.1016/0014-4827(77)90360-3. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Glagov S. Structural integration of the arterial wall. I. Relationships and attachments of medial smooth muscle cells in normally distended and hyperdistended aortas. Lab Invest. 1979 May;40(5):587–602. [PubMed] [Google Scholar]
- FULTON R. M., HUTCHINSON E. C., JONES A. M. Ventricular weight in cardiac hypertrophy. Br Heart J. 1952 Jul;14(3):413–420. doi: 10.1136/hrt.14.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath D., Edwards C., Winson M., Smith P. Effects on the right ventricle, pulmonary vasculature, and carotid bodies of the rat of exposure to, and recovery from, simulated high altitude. Thorax. 1973 Jan;28(1):24–28. doi: 10.1136/thx.28.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hislop A., Haworth S. G., Shinebourne E. A., Reid L. Quantitative structural analysis of pulmonary vessels in isolated ventricular septal defect in infancy. Br Heart J. 1975 Oct;37(10):1014–1021. doi: 10.1136/hrt.37.10.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hislop A., Reid L. Changes in the pulmonary arteries of the rat during recovery from hypoxia-induced pulmonary hypertension. Br J Exp Pathol. 1977 Dec;58(6):653–662. [PMC free article] [PubMed] [Google Scholar]
- Hislop A., Reid L. New findings in pulmonary arteries of rats with hypoxia-induced pulmonary hypertension. Br J Exp Pathol. 1976 Oct;57(5):542–554. [PMC free article] [PubMed] [Google Scholar]
- Jimenez S. A., Yankowski R. Secretion of unhydroxylated chick tendon procollagen. Biochem Biophys Res Commun. 1975 Nov 17;67(2):735–740. doi: 10.1016/0006-291x(75)90874-8. [DOI] [PubMed] [Google Scholar]
- Meyrick B., Reid L. Hypoxia and incorporation of 3H-thymidine by cells of the rat pulmonary arteries and alveolar wall. Am J Pathol. 1979 Jul;96(1):51–70. [PMC free article] [PubMed] [Google Scholar]
- Meyrick B., Reid L. The effect of continued hypoxia on rat pulmonary arterial circulation. An ultrastructural study. Lab Invest. 1978 Feb;38(2):188–200. [PubMed] [Google Scholar]
- Meyrick B., Reid L. Ultrastructural features of the distended pulmonary arteries of the normal rat. Anat Rec. 1979 Jan;193(1):71–97. doi: 10.1002/ar.1091930106. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M., Gamble W., Nadas A. S., Miettinen O. S., Reid L. Rat pulmonary circulation after chronic hypoxia: hemodynamic and structural features. Am J Physiol. 1979 Jun;236(6):H818–H827. doi: 10.1152/ajpheart.1979.236.6.H818. [DOI] [PubMed] [Google Scholar]
- Ross R., Benditt E. P. Wound healing and collagen formation. V. Quantitative electron microscope radioautographic observations of proline-H3 utilization by fibroblasts. J Cell Biol. 1965 Oct;27(1):83–106. doi: 10.1083/jcb.27.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto J., Prockop D. J. Synthesis and secretion of under-hydroxylated procollagen at various temperatures by cells subject to temporary anoxia. Biochem Biophys Res Commun. 1974 Sep 9;60(1):414–423. doi: 10.1016/0006-291x(74)90220-4. [DOI] [PubMed] [Google Scholar]
- WRIGHT B. M. APPARATUS FOR EXPOSING ANIMALS TO REDUCED ATMOSPHERIC PRESSURE FOR LONG PERIODS. Br J Haematol. 1964 Jan;10:75–77. doi: 10.1111/j.1365-2141.1964.tb00680.x. [DOI] [PubMed] [Google Scholar]