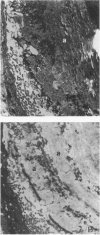
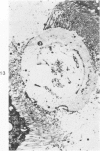
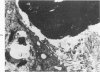
Abstract
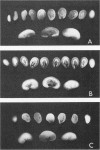
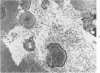
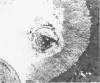
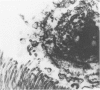
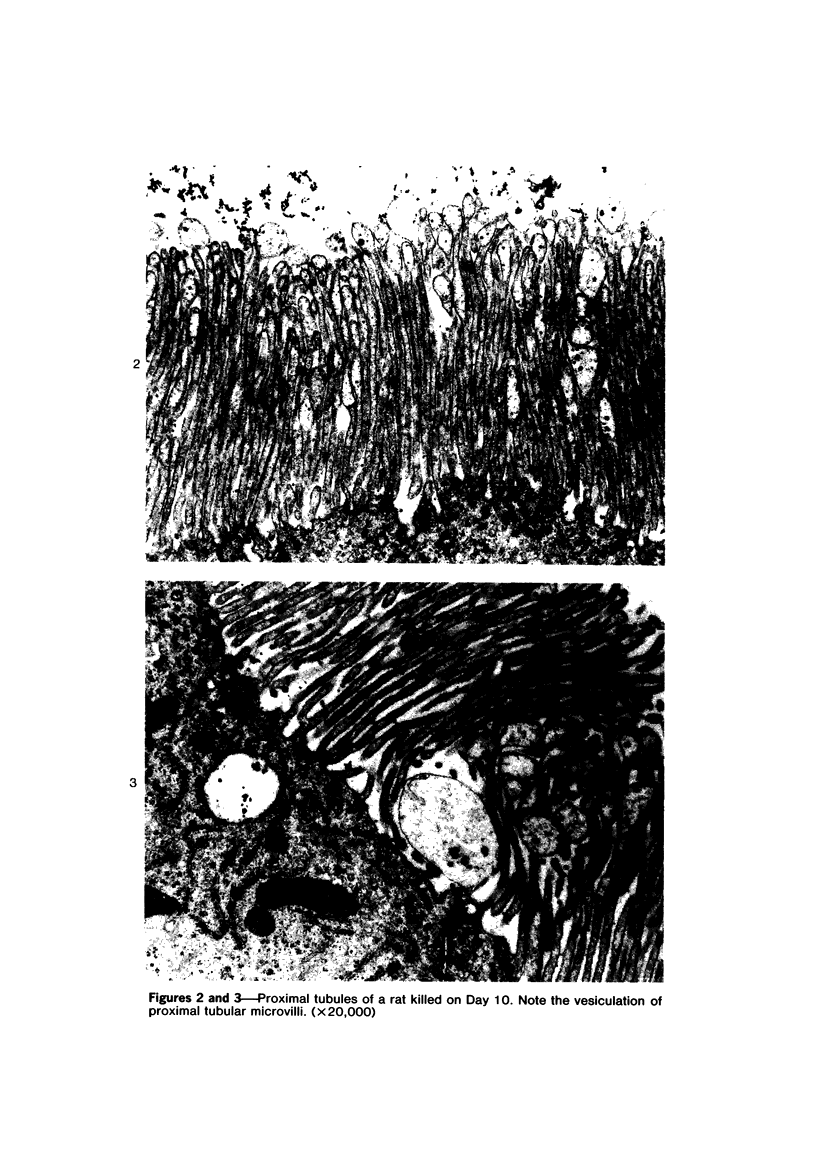
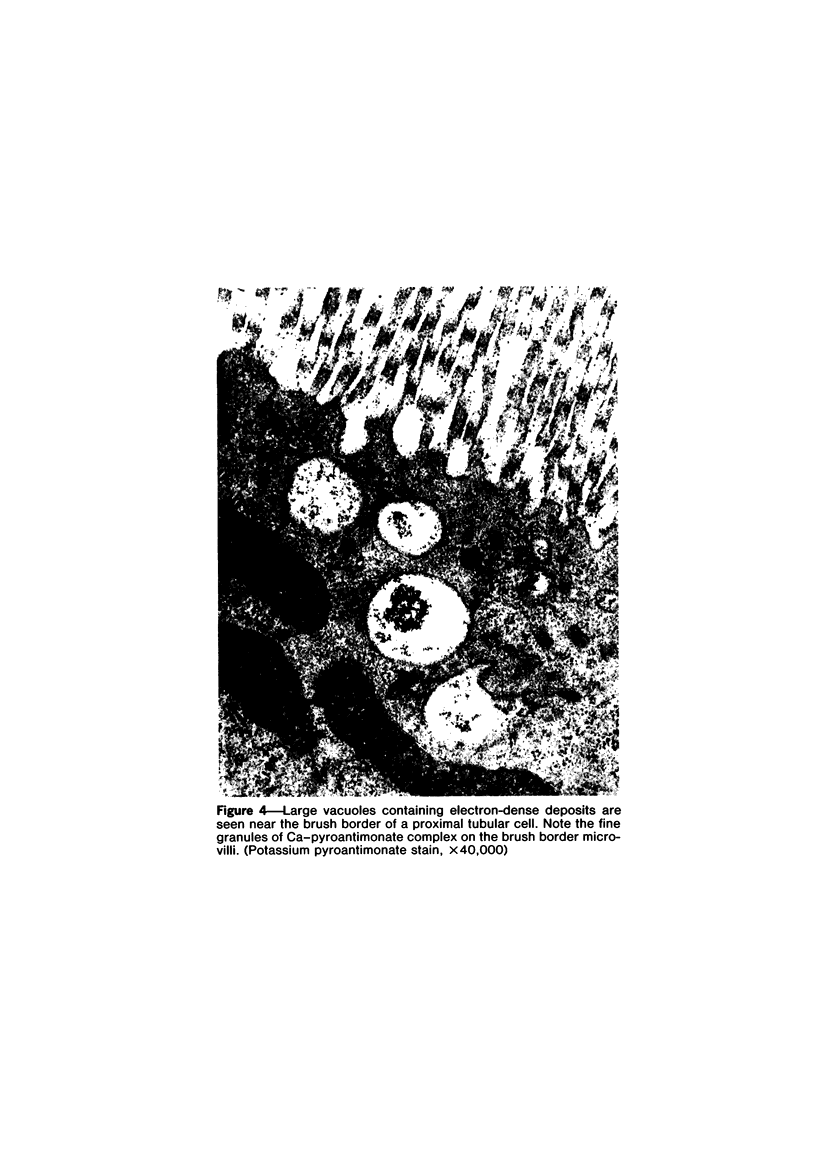
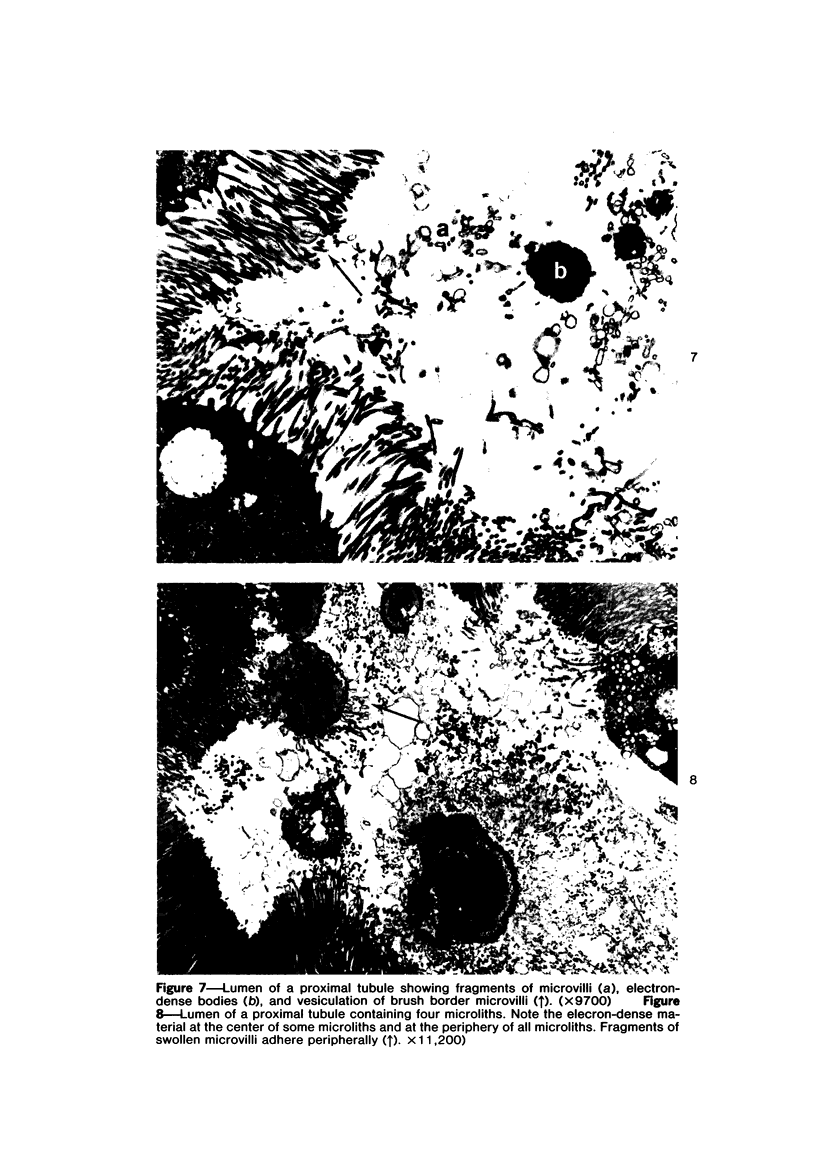
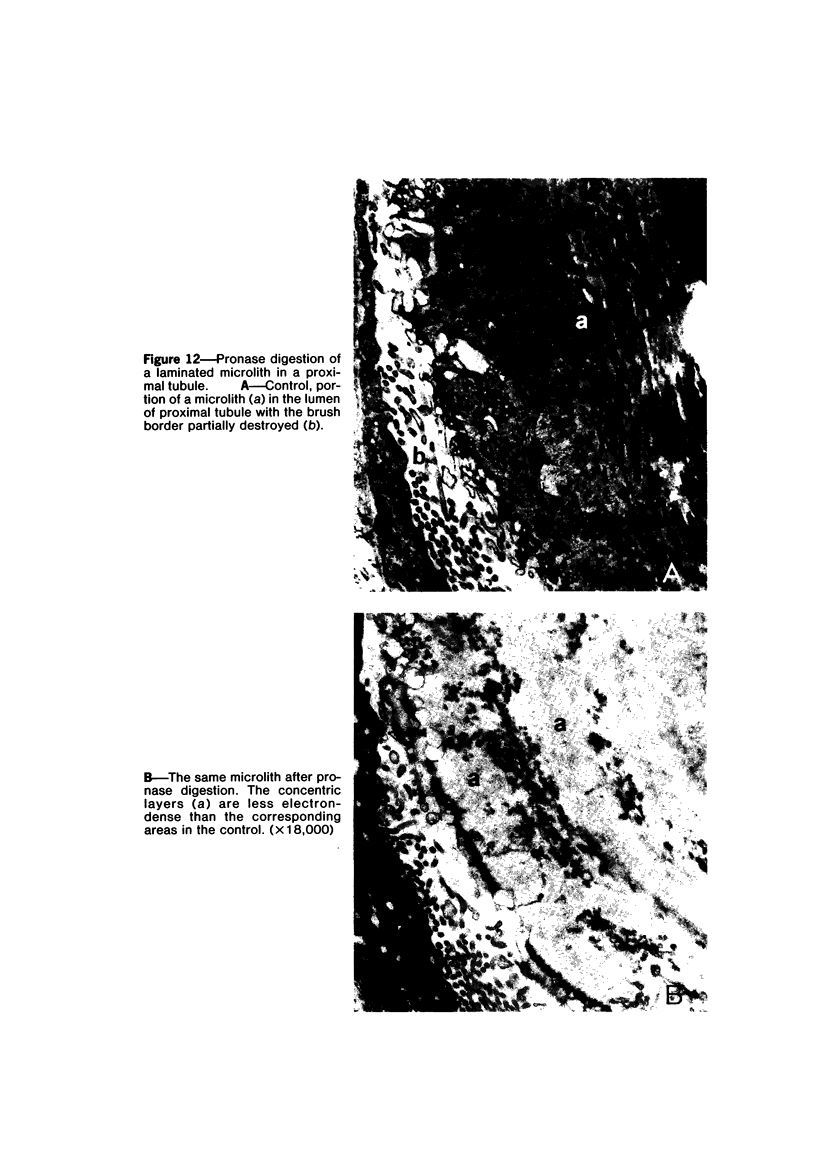
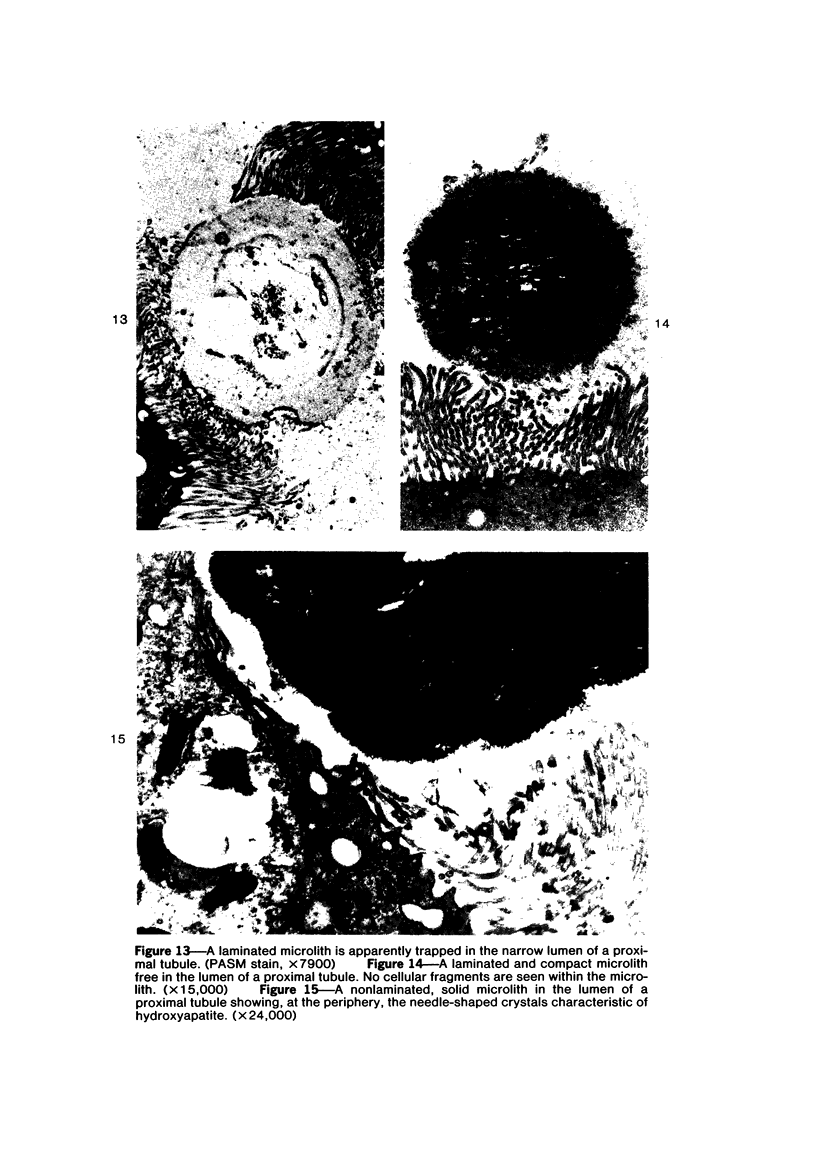
Female Sprague-Dawley rats weighing 45-55 g fed a purified diet for 18 days developed hydroxyapatite intratubular lithiasis, the earliest calcific lesions being detectable by light microscopy on Day 12. The kidneys from these rats revealed ultrastructural changes in proximal tubular cells prior to intraluminal microlith formation. These changes included evidence for increased intracellular calcium, accumulation of electron-dense cytoplasmic granules, and vesiculation and shedding of brush border microvilli within Segment I of the proximal tubule. It was concluded, on the basis of ultrastructural observation, that microvesicles were formed by the shedding of vesiculated microvilli and microvesicles initiated the formation of an intraluminal microurolith in Segment I of the proximal tubule. The initially formed microurolith grew, as it traveled down the nephron, to a size large enough to be visualized by light microscopy. When it reached Segment III (straight segment) of the proximal tubule, the microurolith reached a size so large that it became difficult for it to pass the loop of Henle.
Full text
PDF









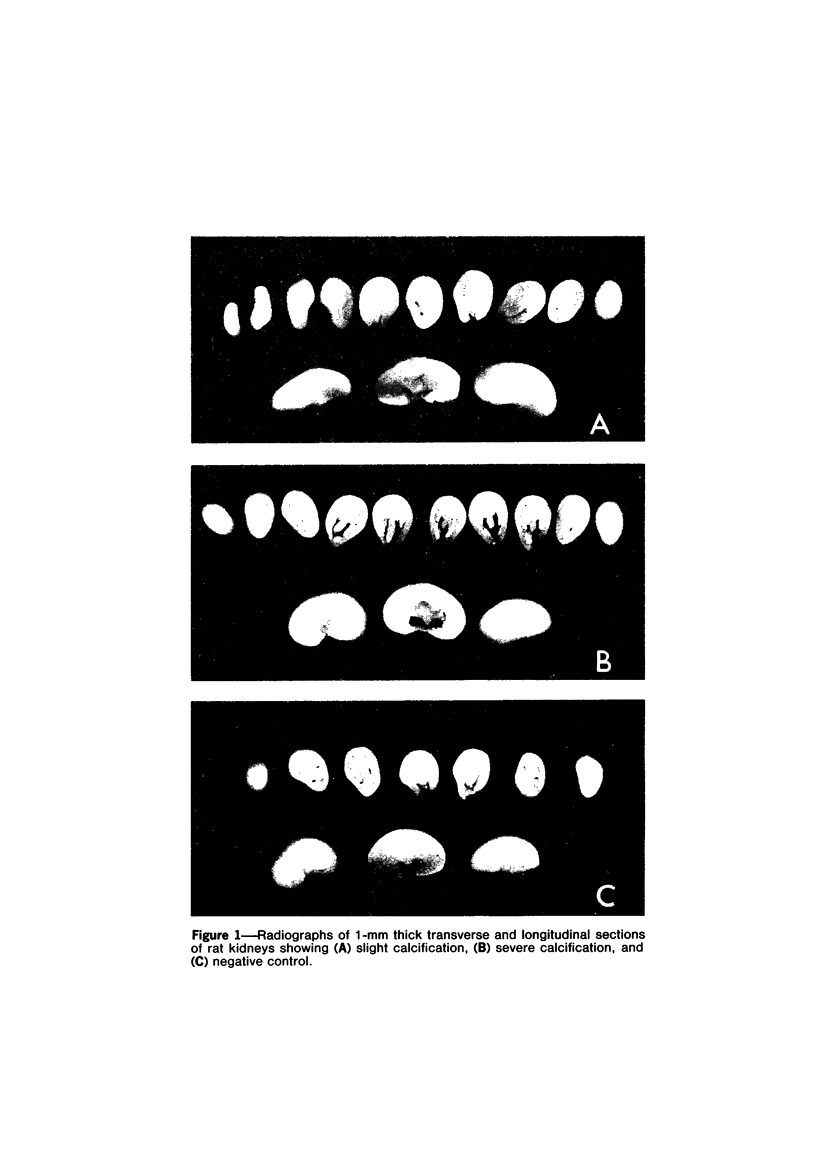


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blagodarov V. N. Ul'trastruktura pochek pri éksperimental'nom kamneobrazovanii i nefrokal'tsinoze. Biull Eksp Biol Med. 1976;81(4):494–496. [PubMed] [Google Scholar]
- Boyce W. H. Organic matrix of human urinary concretions. Am J Med. 1968 Nov;45(5):673–683. doi: 10.1016/0002-9343(68)90203-9. [DOI] [PubMed] [Google Scholar]
- Bronner F., Thompson D. D. Renal transtubular flux of electrolytes in dogs with special reference to calcium. J Physiol. 1961 Jul;157(2):232–250. doi: 10.1113/jphysiol.1961.sp006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAIG J. M. Observations on the kidney after phosphate loading in the rat. Arch Pathol. 1959 Sep;68:306–315. [PubMed] [Google Scholar]
- Cousins F. B., Geary C. P. A sex-determined renal calcification in rats. Nature. 1966 Aug 27;211(5052):980–981. doi: 10.1038/211980b0. [DOI] [PubMed] [Google Scholar]
- De Martino C., Zamboni L. Silver methenamine stain for electron microscopy. J Ultrastruct Res. 1967 Aug;19(3):273–282. doi: 10.1016/s0022-5320(67)80221-1. [DOI] [PubMed] [Google Scholar]
- Debbas G., Hoffman L., Landon E. J., Hurwitz L. Electron microscopic localization of calcium in vascular smooth muscle. Anat Rec. 1975 Aug;182(4):447–471. doi: 10.1002/ar.1091820405. [DOI] [PubMed] [Google Scholar]
- Duffy J. L., Suzuki Y., Churg J. Acute calcium nephropathy. Early proximal tubular changes in the rat kidney. Arch Pathol. 1971 Apr;91(4):340–350. [PubMed] [Google Scholar]
- ENGFELDT B., GARDELL S., HELLSTROM J., IVEMARK B., RHODIN J., STRANDH J. Effect of experimentally induced hyperparathyroidism on renal function and structure. Acta Endocrinol (Copenh) 1958 Sep;29(1):15–26. doi: 10.1530/acta.0.0290015. [DOI] [PubMed] [Google Scholar]
- FORBES R. M. Mineral utilization in the rat. I. Effects of varying dietary ratios of calcium, magnesium and phosphorus. J Nutr. 1963 Jul;80:321–326. doi: 10.1093/jn/80.3.321. [DOI] [PubMed] [Google Scholar]
- Felix R., Fleisch H. Role of matrix vesicles in calcification. Fed Proc. 1976 Feb;35(2):169–171. [PubMed] [Google Scholar]
- Finlayson B. Physicochemical aspects of urolithiasis. Kidney Int. 1978 May;13(5):344–360. doi: 10.1038/ki.1978.53. [DOI] [PubMed] [Google Scholar]
- Fleisch H. Inhibitors and promoters of stone formation. Kidney Int. 1978 May;13(5):361–371. doi: 10.1038/ki.1978.54. [DOI] [PubMed] [Google Scholar]
- GDe Groot A. P., Slump P. Effects of severe alkali treatment of proteins on amino acid composition and nutritive value. J Nutr. 1969 May;98(1):45–56. doi: 10.1093/jn/98.1.45. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Philipsborn D. S., Chen E., Carone F. A. Acute calcium nephrotoxicity. An electron microscopical and semiquantitative light microscopical study. Arch Pathol. 1975 Dec;99(12):650–657. [PubMed] [Google Scholar]
- Geary C. P., Cousins F. B. An oestrogen-linked nephrocalcinosis in rats. Br J Exp Pathol. 1969 Oct;50(5):507–515. [PMC free article] [PubMed] [Google Scholar]
- Goulding A., Malthus R. S. Effect of dietary magnesium on the development of nephrocalcinosis in rats. J Nutr. 1969 Mar;97(3):353–358. doi: 10.1093/jn/97.3.353. [DOI] [PubMed] [Google Scholar]
- Griffith L. D., Bulger R. E., Trump B. F. The ultrastructure of the functioning kidney. Lab Invest. 1967 Feb;16(2):220–246. [PubMed] [Google Scholar]
- Harris C. A., Baer P. G., Chirito E., Dirks J. H. Composition of mammalian glomerular filtrate. Am J Physiol. 1974 Oct;227(4):972–976. doi: 10.1152/ajplegacy.1974.227.4.972. [DOI] [PubMed] [Google Scholar]
- Heroux O., Peter D., Heggtveit A. Long-term effect of suboptimal dietary magnesium on magnesium and calcium contents of organs, on cold tolerance and on lifespan, and its pathological consequences in rats. J Nutr. 1977 Sep;107(9):1640–1652. doi: 10.1093/jn/107.9.1640. [DOI] [PubMed] [Google Scholar]
- Kaunitz H., Johnson R. E. Dietary protein, fat, and minerals in nephrocalcinosis in female rats. Metabolism. 1976 Jan;25(1):69–77. doi: 10.1016/0026-0495(76)90161-x. [DOI] [PubMed] [Google Scholar]
- Kim K. M. Calcification of matrix vesicles in human aortic valve and aortic media. Fed Proc. 1976 Feb;35(2):156–162. [PubMed] [Google Scholar]
- Maunsbach A. B. The influence of different fixatives and fixation methods on the ultrastructure of rat kidney proximal tubule cells. I. Comparison of different perfusion fixation methods and of glutaraldehyde, formaldehyde and osmium tetroxide fixatives. J Ultrastruct Res. 1966 Jun;15(3):242–282. doi: 10.1016/s0022-5320(66)80109-0. [DOI] [PubMed] [Google Scholar]
- Mayahara H., Hirano H., Saito T., Ogawa K. The new lead citrate method for the ultracytochemical demonstration of activity of non-specific alkaline phosphatase (orthophosphoric monoester phosphohydrolase). Histochemie. 1967;11(1):88–96. doi: 10.1007/BF00326615. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch A. L., Anderson H. C. Biogenesis of matrix vesicles in cartilage growth plates. Fed Proc. 1976 Feb;35(2):112–116. [PubMed] [Google Scholar]
- Rambourg A. An improved silver methenamine technique for the detection of periodic acid-reactive complex carbohydrates with the electron microscope. J Histochem Cytochem. 1967 Jul;15(7):409–412. doi: 10.1177/15.7.409. [DOI] [PubMed] [Google Scholar]
- SCARPELLI D. G. EXPERIMENTAL NEPHROCALCINOSIS. A BIOCHEMICAL AND MORPHOLOGIC STUDY. Lab Invest. 1965 Feb;14:123–141. [PubMed] [Google Scholar]
- SCHNEEBERGER E. E., MORRISON A. B. THE NEPHROPATHY OF EXPERIMENTAL MAGNESIUM DEFICIENCY: LIGHT AND ELECTRON MICROSCOPIC INVESTIGATIONS. Lab Invest. 1965 Jun;14:674–686. [PubMed] [Google Scholar]
- Sarkar K., Tohnai G., Levine D. Z. Nephrocalcinosis in chloride depleted rats. An ultrastructural study. Calcif Tissue Res. 1973;12(1):1–7. doi: 10.1007/BF02013716. [DOI] [PubMed] [Google Scholar]
- Spicer S. S., Hardin J. H., Greene W. B. Nuclear precipitates in pyroantimonate-osmium tetroxide-fixed tissues. J Cell Biol. 1968 Oct;39(1):216–221. doi: 10.1083/jcb.39.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struthers B. J., Dahlgren R. R., Hopkins D. T. Biological effects of feeding graded levels of alkali treated soybean protein containing lysinoalanine (N epsilon-2-/carboxyethyl/-L-lysine) in Sprague-Dawley and Wistar rats. J Nutr. 1977 Jul;107(7):1190–1199. doi: 10.1093/jn/107.7.1190. [DOI] [PubMed] [Google Scholar]
- Vermeulen C. W., Lyon E. S. Mechanisms of genesis and growth of calculi. Am J Med. 1968 Nov;45(5):684–692. doi: 10.1016/0002-9343(68)90204-0. [DOI] [PubMed] [Google Scholar]
- Woodard J. C. A morphologic and biochemical study of nutritional nephrocalcinosis in female rats fed semipurified diets. Am J Pathol. 1971 Oct;65(1):253–268. [PMC free article] [PubMed] [Google Scholar]
- Woodard J. C. On the pathogenesis of alpha protein-induced nephrocytomegalia. Lab Invest. 1969 Jan;20(1):9–16. [PubMed] [Google Scholar]
- Woodard J. C. Relationship between the ingredients of semipurified diets and nutritional nephrocalcinosis of rats. Am J Pathol. 1971 Oct;65(1):269–278. [PMC free article] [PubMed] [Google Scholar]
- du Bruyn D. B. A comparison of certain rat strains with respect to susceptibility to nephrocalcinosis. S Afr Med J. 1970 Dec 19;44(49):1417–1418. [PubMed] [Google Scholar]
- du Bruyn D. B. Nephrocalcinosis in the white rat. II. The relationship between dietary magnesium, calcium and phosphorus content and kidney calcification and bone magnesium. S Afr Med J. 1972 Oct 21;46(42):1588–1593. [PubMed] [Google Scholar]
- van Beek L., Feron V. J., de Groot A. P. Nutritional effects of alkali-treated soyprotein in rats. J Nutr. 1974 Dec;104(12):1630–1636. doi: 10.1093/jn/104.12.1630. [DOI] [PubMed] [Google Scholar]