Abstract
A variable population of fat-filled "foam" cells in diet-induced experimental arterial intimal plaques of rabbits and monkeys were analyzed for several features characteristic of macrophages. These included: 1) surface binding and phagocytosis of antibody-coated or complement-coated erythrocytes to detect specific surface receptors; 2) cytochemical tests and ultrastructural features to evaluate cell function and structure; and 3) rapid adherence to glass, a feature of macrophage activity, to isolate and identify a homogeneous population of fat-filled foam cells from excised and disrupted arterial lesions. Mixed populations of cells grown in culture from explants of lesions were also analyzed and lipid-filled cells were studied in histologic sections of adjacent lesions. Eighty to ninety percent of the easily dislodged glass-adherent cells from lesions had surface receptors for the Fc portion of immunoglobulin G and for the third component of complement. Coated red blood cells were readily phagocytized, but noncoated cells were not. Acid lipase activity was demonstrated in the Fc-receptor-positive cells. These cells were also devoid of ultrastructural features of smooth muscle. Among the cells growing or migrating out of explants, a population of large round foam cells possessed all of the macrophage features found in the glass-adherent cells from lesions and lacked ultrastructural characteristics of smooth muscle. Fusiform lipid vacuolated cells also grew out of the explants but did not exhibit surface receptors, failed to phagocytize coated or noncoated erythrocytes and did not stain for acid lipase activity; these cells showed distinctive morphologic features of smooth muscle. In histologic sections of nearby lesions foam cells that showed macrophage characteristics, ie, acid lipase activity and the presence of lysozymelike antigen, lacked ultrastructural smooth muscle features. Smooth muscle cells in lesion sections often contained lipid but demonstrated no lysozyme or acid lipase activity. The occurrence of a population of cells with several functional and structural features of macrophages among the lipid-laden cells of experimental diet-induced arterial lesions suggests that some foam cells may be derived from monocytes. An alternative explanation, that metabolically altered autochthonous arterial wall cells assume one or more characteristics of mononuclear phagocytes is less likely, since some of the markers used in these experiments are unrelated. Both explanations deserve further careful study.
Full text
PDF



















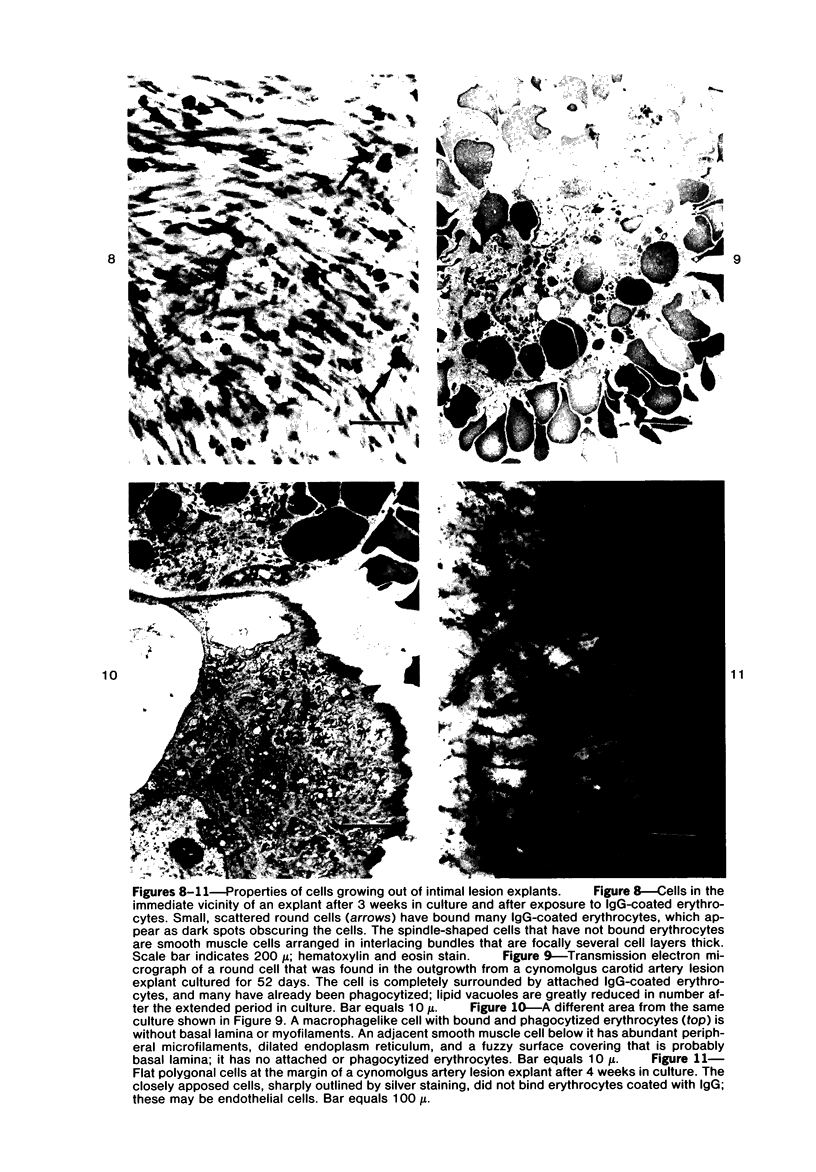
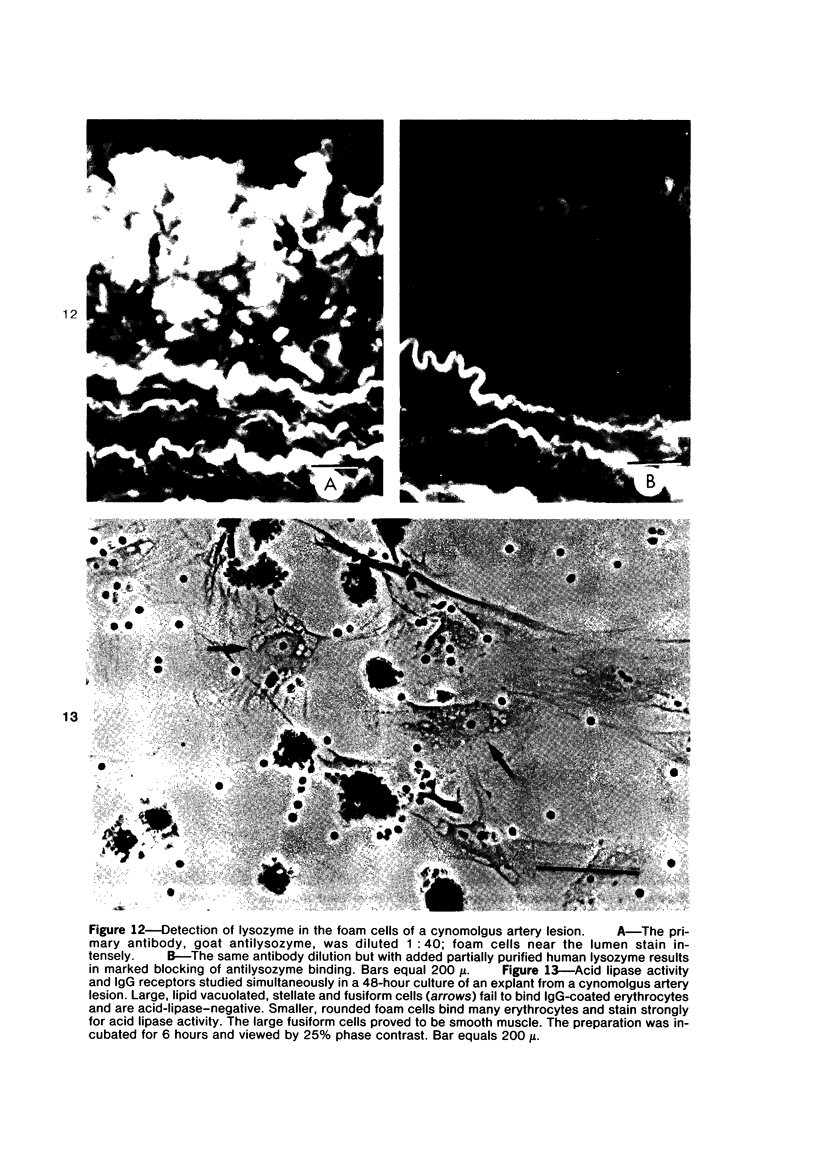
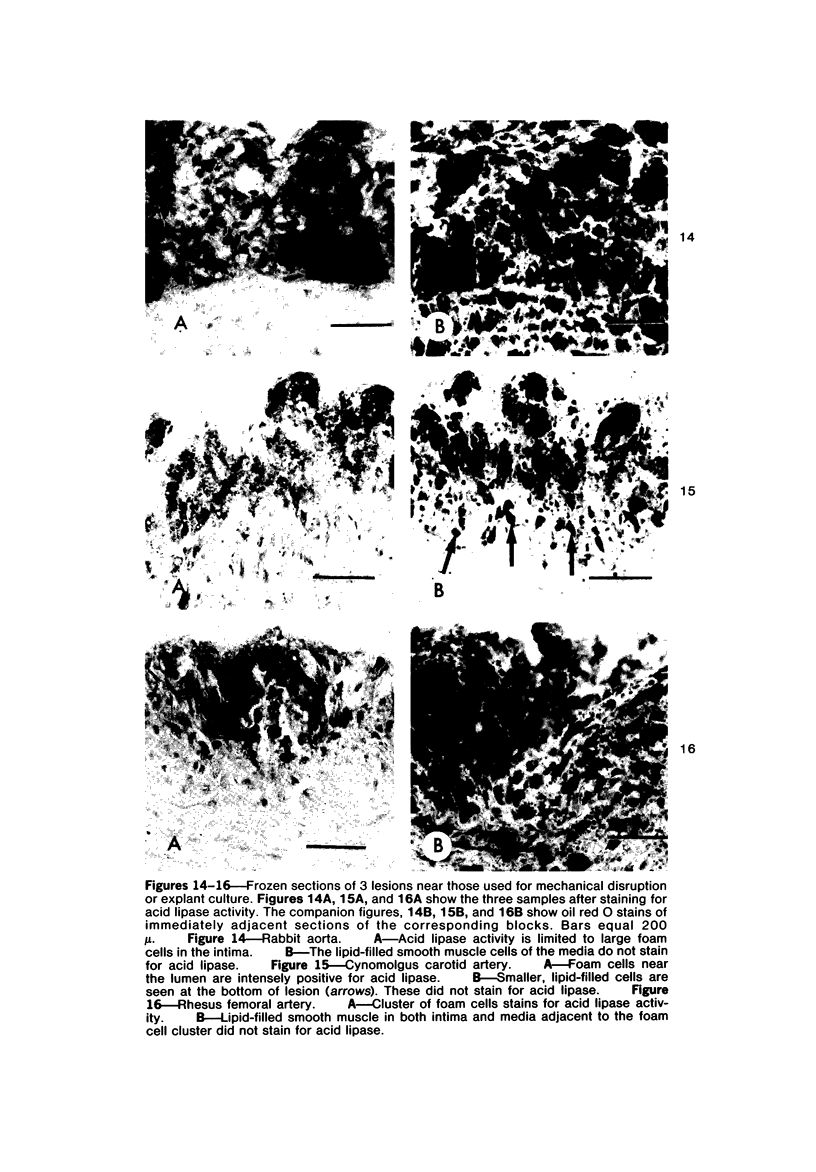

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Bayliss O. B. Detection of macrophages in atherosclerotic lesions with cytochrome oxidase. Br J Exp Pathol. 1976 Feb;57(1):30–36. [PMC free article] [PubMed] [Google Scholar]
- Adams C. W., Bayliss O. B., Turner D. R. Phagocytes, lipid-removal and regression of atheroma. J Pathol. 1975 Aug;116(4):225–238. doi: 10.1002/path.1711160406. [DOI] [PubMed] [Google Scholar]
- Atkinson J. P., Michael J. M., Chaplin H., Jr, Parker C. W. Modulation of macrophage C3b receptor function by cytochalasin-sensitive structures. J Immunol. 1977 Apr;118(4):1292–1299. [PubMed] [Google Scholar]
- Beeson J. H., Wissler R. W. The use of agarose-bound pepsin for the preparation of F(ab')2 fragments of IgG. Immunochemistry. 1977 Apr;14(4):305–312. doi: 10.1016/0019-2791(77)90254-3. [DOI] [PubMed] [Google Scholar]
- Bálint A., Veress B., Nagy Z., Jellinek H. Role of lipophages in the development of rat atheroma. Atherosclerosis. 1972 Jan-Feb;15(1):7–15. doi: 10.1016/0021-9150(72)90033-0. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. I. COMPARATIVE ENZYMOLOGY, ISOLATION, AND PROPERTIES. J Exp Med. 1963 Dec 1;118:991–1008. doi: 10.1084/jem.118.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley-Campbell J., Campbell G. R., Ross R. The smooth muscle cell in culture. Physiol Rev. 1979 Jan;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Breslow J. L., Karnovsky M. J. Regression of myointimal thickening following carotid endothelial injury and development of aortic foam cell lesions in long term hypercholesterolemic rats. Lab Invest. 1977 Jan;36(1):73–81. [PubMed] [Google Scholar]
- Fisher-Dzoga K., Jones R. M., Vesselinovitch D., Wissler R. W. Ultrastructural and immunohistochemical studies of primary cultures of aortic medial cells. Exp Mol Pathol. 1973 Apr;18(2):162–176. doi: 10.1016/0014-4800(73)90014-2. [DOI] [PubMed] [Google Scholar]
- Fowler S., Shio H., Wolinsky H. Subcellular fractionation and morphology of calf aortic smooth muscle cells. Studies on whole aorta, aortic explants, and subcultures grown under different conditions. J Cell Biol. 1977 Oct;75(1):166–184. doi: 10.1083/jcb.75.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEER J. C., McGILL H. C., Jr, STRONG J. P. The fine structure of human atherosclerotic lesions. Am J Pathol. 1961 Mar;38:263–287. [PMC free article] [PubMed] [Google Scholar]
- Gale R. P., Sparkes R. S., Golde D. W. Bone marrow origin of hepatic macrophages (Kupffer cells) in humans. Science. 1978 Sep 8;201(4359):937–938. doi: 10.1126/science.356266. [DOI] [PubMed] [Google Scholar]
- Gaton E., Wolman M. The role of smooth muscle cells and hematogenous macrophages in atheroma. J Pathol. 1977 Oct;123(2):123–128. doi: 10.1002/path.1711230208. [DOI] [PubMed] [Google Scholar]
- Geer J. C. Fine structure of human aortic intimal thickening and fatty streaks. Lab Invest. 1965 Oct;14(10):1764–1783. [PubMed] [Google Scholar]
- Gerrity R. G., Naito H. K., Richardson M., Schwartz C. J. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. Am J Pathol. 1979 Jun;95(3):775–792. [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Bialecki H., Zetter B. R. Factors involved in the modulation of cell proliferation in vivo and in vitro: the role of fibroblast and epidermal growth factors in the proliferative response of mammalian cells. In Vitro. 1978 Jan;14(1):85–118. doi: 10.1007/BF02618177. [DOI] [PubMed] [Google Scholar]
- HAUST M. D., MORE R. H., MOVAT H. Z. The mechanism of fibrosis in arteriosclerosis. Am J Pathol. 1959 Mar-Apr;35(2):265–273. [PMC free article] [PubMed] [Google Scholar]
- Haley N. J., Shio H., Fowler S. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. I. Resolution of aortic cell populations by metrizamide density gradient centrifugation. Lab Invest. 1977 Sep;37(3):287–296. [PubMed] [Google Scholar]
- Hartwig J. H., Stossel T. P. Isolation and properties of actin, myosin, and a new actinbinding protein in rabbit alveolar macrophages. J Biol Chem. 1975 Jul 25;250(14):5696–5705. [PubMed] [Google Scholar]
- Howard J. G., Benacerraf B. Properties of macrophage receptors for cytophilic antibodies. Br J Exp Pathol. 1966 Apr;47(2):193–200. [PMC free article] [PubMed] [Google Scholar]
- Imai H., Lee K. T., Pastori S., Panlilio E., Florentin R., Thomas W. A. Atherosclerosis in rabbits. Architectural and subcellular alterations of smooth muscle cells of aortas in response to hyperlipemia. Exp Mol Pathol. 1966 Jun;5(3):273–310. doi: 10.1016/0014-4800(66)90036-0. [DOI] [PubMed] [Google Scholar]
- Jones R. M., Schaffner T. J., Chassagne G., Glagov S., Wissler R. W. Comparison of coronary with aortic fatty streaks in rhesus monkeys. Scan Electron Microsc. 1979;(3):829–834. [PubMed] [Google Scholar]
- KARNOVSKY M. L. Metabolic basis of phagocytic activity. Physiol Rev. 1962 Jan;42:143–168. doi: 10.1152/physrev.1962.42.1.143. [DOI] [PubMed] [Google Scholar]
- Kerbel R. S., Davies A. J. The possible biological significance of Fc receptors on mammalian lymphocytes and tumor cells. Cell. 1974 Oct;3(2):105–112. doi: 10.1016/0092-8674(74)90113-5. [DOI] [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Receptors for complement of leukocytes. J Exp Med. 1968 Nov 1;128(5):991–1009. doi: 10.1084/jem.128.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- MYRVIK Q. N., LEAKE E. S., FARISS B. Lysozyme content of alveolar and peritoneal macrophages from the rabbit. J Immunol. 1961 Feb;86:133–136. [PubMed] [Google Scholar]
- Newman H. A., Murad T. M., Geer J. C. Foam cells of rabbit atheromatous lesion. Identification and cholesterol uptake in isolated cells. Lab Invest. 1971 Dec;25(6):586–595. [PubMed] [Google Scholar]
- POOLE J. C., FLOREY H. W. Changes in the endothelium of the aorta and the behaviour of macrophages in experimental atheroma of rabbits. J Pathol Bacteriol. 1958 Apr;75(2):245–251. doi: 10.1002/path.1700750202. [DOI] [PubMed] [Google Scholar]
- Parker F. An Electron Microscopic Study of Experimental Atherosclerosis. Am J Pathol. 1960 Jan;36(1):19–53. [PMC free article] [PubMed] [Google Scholar]
- Peterson M., Day A. J., Tume R. K., Eisenberg E. Ultrastructure, fatty acid content, and metabolic activity of foam cells and other fractions separated from rabbit atherosclerotic lesions. Exp Mol Pathol. 1971 Oct;15(2):157–169. doi: 10.1016/0014-4800(71)90096-7. [DOI] [PubMed] [Google Scholar]
- Reaven E. P., Axline S. G. Subplasmalemmal microfilaments and microtubules in resting and phagocytizing cultivated macrophages. J Cell Biol. 1973 Oct;59(1):12–27. doi: 10.1083/jcb.59.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner T., Elner V. M., Bauer M., Wissler R. W. Acid lipase: a histochemical and biochemical study using triton X100-naphtyl palmitate micelles. J Histochem Cytochem. 1978 Sep;26(9):696–712. doi: 10.1177/26.9.30799. [DOI] [PubMed] [Google Scholar]
- Spicer S. S., Frayser R., Virella G., Hall B. J. Immunocytochemical localization of lysozymes in respiratory and other tissues. Lab Invest. 1977 Mar;36(3):282–295. [PubMed] [Google Scholar]
- Stary H. C., Strong J. P. The fine structure of nonatherosclerotic intimal thickening, of developing, and of regressing atherosclerotic lesions at the bifurcation of the left coronary artery. Adv Exp Med Biol. 1976;67(00):89–108. doi: 10.1007/978-1-4614-4618-7_5. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. Phagocytosis: recognition and ingestion. Semin Hematol. 1975 Jan;12(1):83–116. [PubMed] [Google Scholar]
- Streuli R. A., Chung J., Scanu A. M., Yachnin S. Inhibition of human lymphocyte E-rosette formation by oxygenated sterols. J Immunol. 1979 Dec;123(6):2897–2902. [PubMed] [Google Scholar]
- Taylor K., Schaffner T., Wissler R. W., Glagov S. Immuno-morphologic identification and characterization of cells derived from experimental atherosclerotic lesions. Scan Electron Microsc. 1979;(3):815–822. [PubMed] [Google Scholar]
- Thomas E. D., Ramberg R. E., Sale G. E., Sparkes R. S., Golde D. W. Direct evidence for a bone marrow origin of the alveolar macrophage in man. Science. 1976 Jun 4;192(4243):1016–1018. doi: 10.1126/science.775638. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Secretory function of mononuclear phagocytes: a review. Am J Pathol. 1976 May;83(2):396–418. [PMC free article] [PubMed] [Google Scholar]
- Wissler R. W., Vesselinovitch D. Experimental models of human atherosclerosis. Ann N Y Acad Sci. 1968 Nov 21;149(2):907–922. [PubMed] [Google Scholar]
- Wissler R. W., Vesselinovitch D., Getz G. S. Abnormalities of the arterial wall and its metabolism in atherogenesis. Prog Cardiovasc Dis. 1976 Mar-Apr;18(5):341–369. doi: 10.1016/0033-0620(76)90002-5. [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D., Grusky G., Marcus A. Transformation of monocytes into "fat" cells. Lab Invest. 1978 May;38(5):620–628. [PubMed] [Google Scholar]
- van Furth R. An approach to the characterization of mononuclear phagocytes involved in pathological processes. Agents Actions. 1976 Feb;6(1-3):91–98. doi: 10.1007/BF01972190. [DOI] [PubMed] [Google Scholar]










