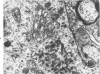Abstract
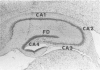
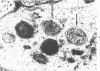
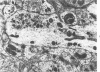
The ultrastructural cytopathologic and cytochemical effects of trimethyltin (TMT) neurotoxicity were delineated in hippocampal and pyriform neurons of acutely intoxicated adult rats. TMT produced neuronal necrosis that preferentially involved hippocampal formation pyriform cortex. The first subcellular alterations were multifocal collection of dense-cored vesicles and tubules and membrane-delimited vacuoles in the cytoplasm of the perikaryon and proximal dendrite. Ultrastructural cytochemical examination revealed that the vesicles and tubules had acid phosphatase activity analagous to Golgi-associated endoplasmic reticulum (GERL). Shortly after the appearance of the GERL-like vesicles and tubules, autophagic vacuoles and polymorphic dense bodies accumulated in the neuronal cytoplasm. Some dense bodies appeared to arise from the dense-cored tubules. Neuronal necrosis was characterized by increased electron density of the cytoplasm and large, electron-dense intranuclear masses. Alterations of mitochondria and other organelles were not observed in the early stages of cell injury. No light- or electron-microscopic alterations were found in liver or kidney. Comparable subcellular alterations were observed in adult and neonatal rats chronically intoxicated with TMT. A series of other trialkyl and tricyclic tins and dimethyltin did not produce similar pathologic findings. The GERL-like accumulations are unique in neuronal cytopathology. These findings suggests that GERL and autophagy play an important role in the pathogenesis of TMT-induced neuronal injury.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge W. N., Street B. W., Skilleter D. N. Oxidative phosphorylation. Halide-dependent and halide-independent effects of triorganotin and trioganolead compounds on mitochondrial functions. Biochem J. 1977 Dec 15;168(3):353–364. doi: 10.1042/bj1680353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleu F. P., Katzman R., Terry R. D. Fine structure and electrolyte analyses of cerebral edema induced by alkyl tin intoxication. J Neuropathol Exp Neurol. 1963 Jul;22(3):403–413. doi: 10.1097/00005072-196307000-00003. [DOI] [PubMed] [Google Scholar]
- BARNES J. M., STONER H. B. Toxic properties of some dialkyl and trialkyl tin salts. Br J Ind Med. 1958 Jan;15(1):15–22. doi: 10.1136/oem.15.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaker W. D., Krigman M. R., Thomas D. J., Mushak P., Morell P. Effect of triethyl tin on myelination in the developing rat. J Neurochem. 1981 Jan;36(1):44–52. doi: 10.1111/j.1471-4159.1981.tb02375.x. [DOI] [PubMed] [Google Scholar]
- Brown A. W., Aldridge W. N., Street B. W., Verschoyle R. D. The behavioral and neuropathologic sequelae of intoxication by trimethyltin compounds in the rat. Am J Pathol. 1979 Oct;97(1):59–82. [PMC free article] [PubMed] [Google Scholar]
- Brown A. W., Levy D. E., Kublik M., Harrow J., Plum F., Brierley J. B. Selective chromatolysis of neurons in the gerbil brain: a possible consequence of "epileptic" activity produced by common carotid artery occlusion. Ann Neurol. 1979 Feb;5(2):127–138. doi: 10.1002/ana.410050206. [DOI] [PubMed] [Google Scholar]
- Bubis J. J., Fujimoto T., Ito U., Mrsulja B. J., Spatz M., Klatzo I. Experimental cerebral ischemia in Mongolian gerbils. v. Ultrastructural changes in H3 sector of the hippocampus. Acta Neuropathol. 1976 Nov 15;36(3):285–294. doi: 10.1007/BF00685372. [DOI] [PubMed] [Google Scholar]
- CREMER J. E. Biochemical studies on the toxicity of tetraethyl lead and other organo-lead compounds. Br J Ind Med. 1959 Jul;16:191–199. doi: 10.1136/oem.16.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREMER J. E. The biochemistry of organotin compounds; the conversion of tetraethyltin into triethyltin in mammals. Biochem J. 1958 Apr;68(4):685–692. doi: 10.1042/bj0680685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker R. S. Lysosomal packaging in differentiating and degenerating anuran lateral motor column neurons. J Cell Biol. 1974 Jun;61(3):599–612. doi: 10.1083/jcb.61.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn D., Lüllmann-Rauch R. Drug-induced experimental lipidosis in the nervous system. Neuroscience. 1979;4(6):697–612. doi: 10.1016/0306-4522(79)90001-0. [DOI] [PubMed] [Google Scholar]
- Hand A. R. Cytochemical differentiation of the Golgi apparatus from GERL. J Histochem Cytochem. 1980 Jan;28(1):82–86. doi: 10.1177/28.1.7351475. [DOI] [PubMed] [Google Scholar]
- Holtzman E., Novikoff A. B., Villaverde H. Lysosomes and GERL in normal and chromatolytic neurons of the rat ganglion nodosum. J Cell Biol. 1967 May;33(2):419–435. doi: 10.1083/jcb.33.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito U., Spatz M., Walker J. T., Jr, Klatzo I. Experimental cerebral ischemia in mongolian gerbils. I. Light microscopic observations. Acta Neuropathol. 1975 Aug 27;32(3):209–223. doi: 10.1007/BF00696570. [DOI] [PubMed] [Google Scholar]
- Laatsch R. H., Cowan W. M. Electron microscopic studies of the dentate gyrus of the rat. I. Normal structure with special reference to synaptic organization. J Comp Neurol. 1966 Nov;128(3):359–395. doi: 10.1002/cne.901280305. [DOI] [PubMed] [Google Scholar]
- Langford L. A., Coggeshall R. E. The use of potassium ferricyanide in neural fixation. Anat Rec. 1980 Jul;197(3):297–303. doi: 10.1002/ar.1091970304. [DOI] [PubMed] [Google Scholar]
- Lapresle J., Annabi A. Olivopontocerebellar atrophy with velopharyngolaryngeal paralysis: a contribution to the somatotopy of the nucleus ambiguus. J Neuropathol Exp Neurol. 1979 Jul;38(4):401–406. doi: 10.1097/00005072-197907000-00005. [DOI] [PubMed] [Google Scholar]
- Meldrum B. S., Brierley J. B. Prolonged epileptic seizures in primates. Ischemic cell change and its relation to ictal physiological events. Arch Neurol. 1973 Jan;28(1):10–17. doi: 10.1001/archneur.1973.00490190028002. [DOI] [PubMed] [Google Scholar]
- NOVIKOFF A. B., GOLDFISCHER S. Nucleosidediphosphatase activity in the Golgi apparatus and its usefulness for cytological studies. Proc Natl Acad Sci U S A. 1961 Jun 15;47:802–810. doi: 10.1073/pnas.47.6.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M. Cytochemical contributions to differentiating GERL from the Golgi apparatus. Histochem J. 1977 Sep;9(5):525–551. doi: 10.1007/BF01002901. [DOI] [PubMed] [Google Scholar]
- Novikoff P. M., Novikoff A. B., Quintana N., Hauw J. J. Golgi apparatus, GERL, and lysosomes of neurons in rat dorsal root ganglia, studied by thick section and thin section cytochemistry. J Cell Biol. 1971 Sep;50(3):859–886. doi: 10.1083/jcb.50.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavola L. G. The corpus luteum of the guinea pig. III. Cytochemical studies on the Golgi complex and GERL during normal postpartum regression of luteal cells, emphasizing the origin of lysosomes and autophagic vacuoles. J Cell Biol. 1978 Oct;79(1):59–73. doi: 10.1083/jcb.79.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quatacker J. R. Different aspects of membrane differentiation at the inner side (GERL) of the Golgi apparatus in rabbit luteal cells. Histochem J. 1979 Jul;11(4):399–416. doi: 10.1007/BF01002768. [DOI] [PubMed] [Google Scholar]