Abstract
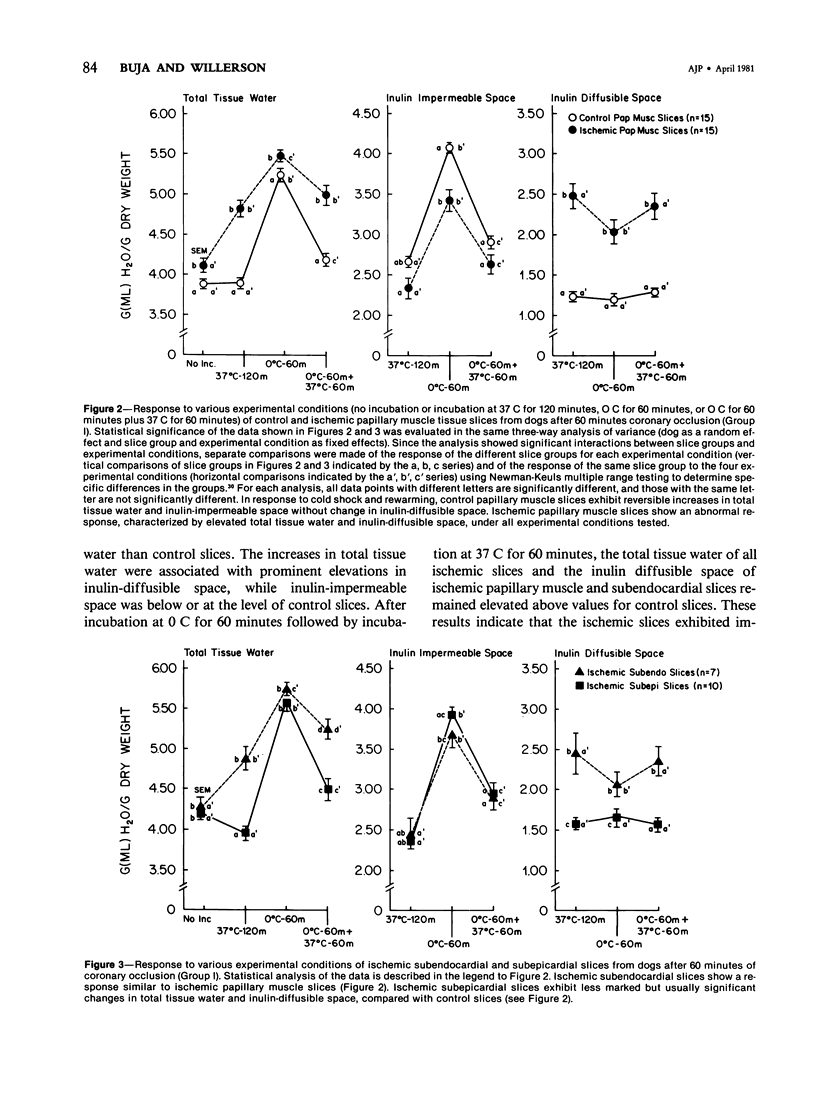
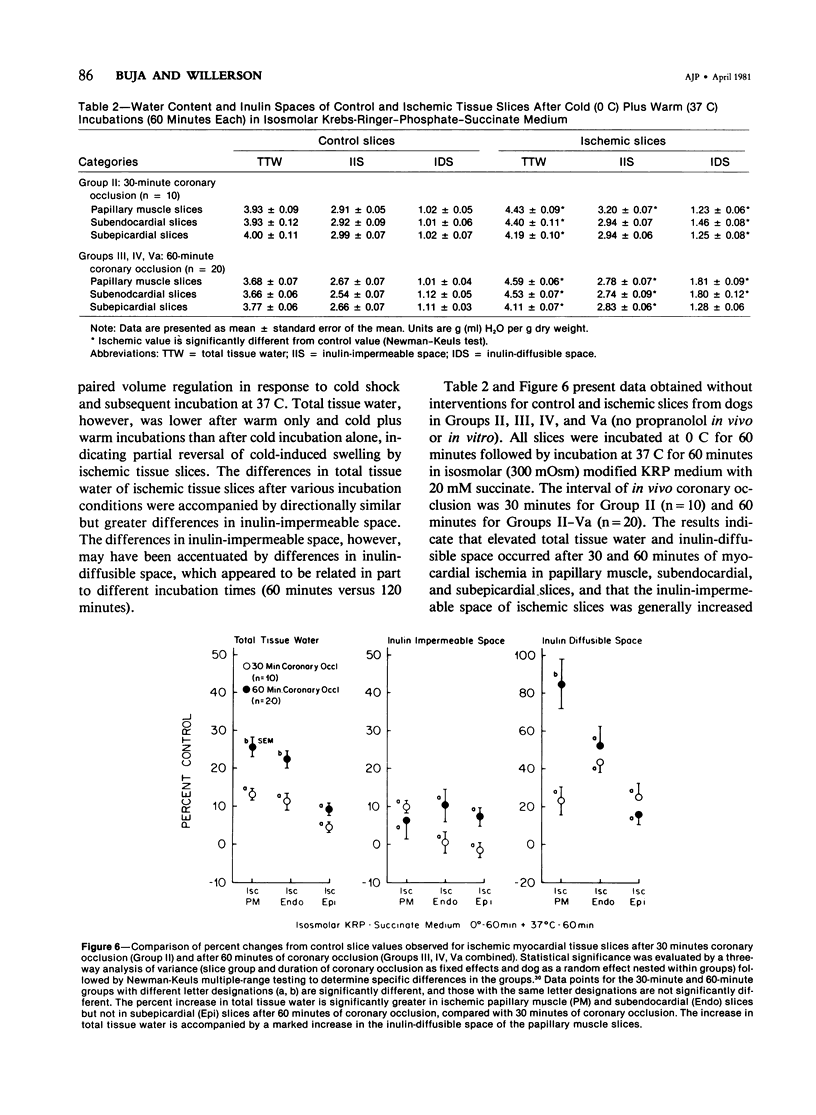
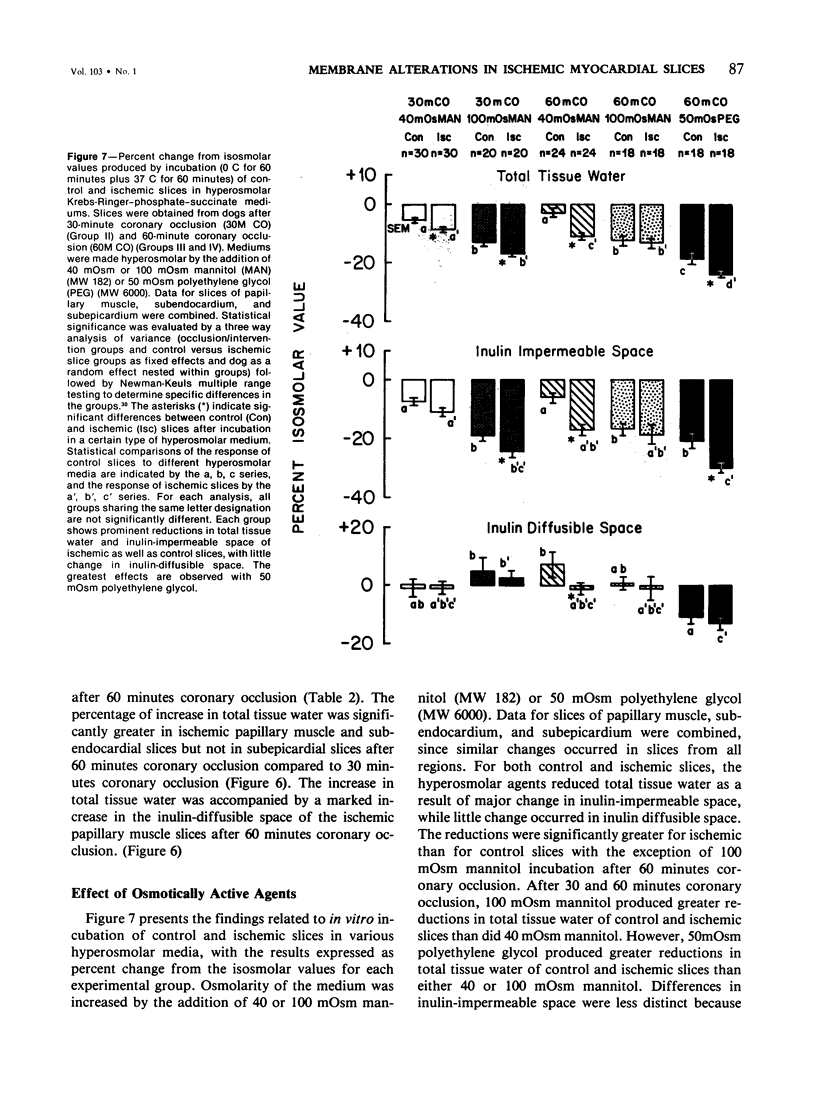
The authors used an in vitro myocardial tissue slice technique to quantitate the transmural distribution of alterations in cell volume regulation and membrane integrity following early ischemic injury and to evaluate directly the effects of therapeutic interventions in a system not subjects to influences of coronary blood flow. Left circumflex coronary occlusion was produced in 57 dogs for 30 or 60 minutes. After in vitro incubation in Krebs-Ringer-phosphate-succinate medium containing trace 14C-inulin, typical values (ml H2O/g dry weight) for control nonischemic myocardial slices were 3.68 +/- 0.07 (SEM) for total tissue water, 2.67 +/- 0.07 for inulin impermeable space, and 1.01 +/- 0.04 for inulin diffusible space. Ischemic myocardial slices exhibited an impaired response to cold shock (0 C for 60 minutes) and rewarming (37 C for 60 minutes). After 60 minutes coronary occlusion, respective increases in total tissue water, inulin-impermeable space and inulin-diffusible space of ischemic slices were 25.5 +/- 2.6%, 6.2 +/- 4.9% and 84.4 +/- 12.5% for papillary muscle, 22.2 +/- 2.1%, 10.4 +/- 4.2% and 52.5 %/- 10.3% for subendocardium and 9.1 +/- 1.5%, 7.2 +/- 2.3% and 15.8 +/- 5.5% for subepicardium. Significant but usually less marked alterations occurred after 30 minutes of coronary occlusion. Propranolol treatment in vivo (2 mg/kg) and/or in vitro (0.01 mg/ml medium) produced no significant changes in tissue water or inulin spaces of ischemic slices, compared with saline controls. Incubation in hyperosmolar mediums resulted in significant reductions in total tissue water and inulin-impermeable space with little change in inulin-diffusible space of both ischemic and control slices. Fifty milliosmolar polyethylene glycol (MW 6000) produced a greater reduction in tissue water and ultrastructural evidence of cell swelling than did either 40 or 100 milliosmolar mannitol (MW 182). The major effect of hyperosmolar incubation appeared to be a selective reduction in edema of cells with structurally intact membranes. Thus, in vitro studies, with myocardial tissue slices provide evidence of widespread alterations of membrane integrity after 30--60 minutes of in vivo coronary artery occlusion. In vitro abnormalities of cell volume regulation can be partially reversed by direct osmotic effects on myocardial cells.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beller G. A., Conroy J., Smith T. W. Ischemia-induced alterations in myocardial (Na+ + K+)-ATPase and cardiac glycoside binding. J Clin Invest. 1976 Feb;57(2):341–350. doi: 10.1172/JCI108285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton K. P., Hagler H. K., Templeton G. H., Willerson J. T., Buja L. M. Lanthanum probe studies of cellular pathophysiology induced by hypoxia in isolated cardiac muscle. J Clin Invest. 1977 Dec;60(6):1289–1302. doi: 10.1172/JCI108888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton K. P., Templeton G. H., Hagler H. K., Willerson J. T., Buja L. M. Effect of glucose availability on functional membrane integrity, ultrastructure and contractile performance following hypoxia and reoxygenation in isolated feline cardiac muscle. J Mol Cell Cardiol. 1980 Jan;12(1):109–133. doi: 10.1016/0022-2828(80)90114-5. [DOI] [PubMed] [Google Scholar]
- Caulfield J. B., Willerson J. T., Weisfeldt M. L., Powell W. J., Jr Effect of mannitol on mitochondrial morphology in hypoxic myocardium. Recent Adv Stud Cardiac Struct Metab. 1973;3:753–762. [PubMed] [Google Scholar]
- Chien K. R., Abrams J., Serroni A., Martin J. T., Farber J. L. Accelerated phospholipid degradation and associated membrane dysfunction in irreversible, ischemic liver cell injury. J Biol Chem. 1978 Jul 10;253(13):4809–4817. [PubMed] [Google Scholar]
- DiBona D. R., Powell W. J., Jr Quantitative correlation between cell swelling and necrosis in myocardial ischemia in dogs. Circ Res. 1980 Nov;47(5):653–665. doi: 10.1161/01.res.47.5.653. [DOI] [PubMed] [Google Scholar]
- Fixler D. E., Buja L. M., Wheeler J. M., Willerson J. T. Influence of mannitol on maintaining coronary flows and salvaging myocardium during ventriculotomy and during prolonged coronary artery ligation. Circulation. 1977 Sep;56(3):340–346. doi: 10.1161/01.cir.56.3.340. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Jennings R. B., Hill M. L., Grochowski E. Experimental myocardial ischemic injury. II. Effect of in vivo ischemia on dog heart slice function in vitro. J Mol Cell Cardiol. 1976 Mar;8(3):189–204. doi: 10.1016/0022-2828(76)90036-5. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Seabra-Gomes R., Nayler W. G., Jennings R. B. Irreversible myocardial injury in anoxic perfused rat hearts. Am J Pathol. 1975 Sep;80(3):419–450. [PMC free article] [PubMed] [Google Scholar]
- Ganote C. E., Worstell J., Iannotti J. P., Kaltenbach J. P. Cellular swelling and irreversible myocardial injury. Effects of polyethylene glycol and mannitol in perfused rat hearts. Am J Pathol. 1977 Jul;88(1):95–118. [PMC free article] [PubMed] [Google Scholar]
- Grochowski E. C., Ganote C. E., Hill M. L., Jennings R. B. Experimental myocardial ischemic injury. I. A comparison of Stadie-Riggs and free-hand slicing techniques on tissue ultrastructure, water and electrolytes during in vitro incubation. J Mol Cell Cardiol. 1976 Mar;8(3):173–187. doi: 10.1016/0022-2828(76)90035-3. [DOI] [PubMed] [Google Scholar]
- Hagler H. K., Sherwin L., Buja L. M. Effect of different methods of tissue preparation on mitochondrial inclusions of ischemic and infarcted canine myocardium: transmission and analytic electron microscopic study. Lab Invest. 1979 May;40(5):529–544. [PubMed] [Google Scholar]
- Hems R., Stubbs M., Krebs H. A. Restricted permeability of rat liver for glutamate and succinate. Biochem J. 1968 May;107(6):807–815. doi: 10.1042/bj1070807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirzel H. O., Kirk E. S. The effect of mannitol following permanent coronary occlusion. Circulation. 1977 Dec;56(6):1006–1015. doi: 10.1161/01.cir.56.6.1006. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Hawkins H. K., Lowe J. E., Hill M. L., Klotman S., Reimer K. A. Relation between high energy phosphate and lethal injury in myocardial ischemia in the dog. Am J Pathol. 1978 Jul;92(1):187–214. [PMC free article] [PubMed] [Google Scholar]
- Jolly W. W., Wilhelm D. D., Harris R. A. Assessment of tissue and cell damage by succinate oxidation. J Mol Cell Cardiol. 1979 May;11(5):485–500. doi: 10.1016/0022-2828(79)90472-3. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Fishbein M. C., Cotran R. S., Braunwald E., Maroko P. R. The effect of propranolol on microvascular injury in acute myocardial ischemia. Circulation. 1977 Jun;55(6):872–880. doi: 10.1161/01.cir.55.6.872. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Reimer K. A., Willerson J. T., Jennings R. B. Reduction of experimental myocardial infarct size with hyperosmolar mannitol. Proc Soc Exp Biol Med. 1976 Apr;151(4):677–683. doi: 10.3181/00379727-151-39285. [DOI] [PubMed] [Google Scholar]
- Krishnamurty V. S., Adams H. R., Smitherman T. C., Templeton G. H., Willerson J. T. Influence of mannitol on contractile responses of isolated perfused arteries. Am J Physiol. 1977 Jan;232(1):H59–H66. doi: 10.1152/ajpheart.1977.232.1.H59. [DOI] [PubMed] [Google Scholar]
- Krishnamurty V. S., Adams H. R., Templeton G. H., Willerson J. T. Inhibitory effect of hypertonic mannitol on vasoconstrictor and vasodilator responses of isolated coronary arteries. Am J Physiol. 1978 Dec;235(6):H728–H735. doi: 10.1152/ajpheart.1978.235.6.H728. [DOI] [PubMed] [Google Scholar]
- Leaf A. Regulation of intracellular fluid volume and disease. Am J Med. 1970 Sep;49(3):291–295. doi: 10.1016/s0002-9343(70)80019-5. [DOI] [PubMed] [Google Scholar]
- MORALES AGUILERA A., VAUGHANWILLIAMS E. M. THE EFFECTS ON CARDIAC MUSCLE OF BETA-RECEPTOR ANTAGONISTS IN RELATION TO THEIR ACTIVITY AS LOCAL ANAESTHETICS. Br J Pharmacol Chemother. 1965 Apr;24:332–338. doi: 10.1111/j.1476-5381.1965.tb01719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight A. D., Leaf A. Regulation of cellular volume. Physiol Rev. 1977 Jul;57(3):510–573. doi: 10.1152/physrev.1977.57.3.510. [DOI] [PubMed] [Google Scholar]
- Miura M., Thomas R., Ganz W., Sokol T., Shell W. E., Toshimitsu T., Kwan A. C., Singh B. N. The effect of delay in propranolol administration on reduction of myocardial infarct size after experimental coronary artery occlusion in dogs. Circulation. 1979 Jun;59(6):1148–1157. doi: 10.1161/01.cir.59.6.1148. [DOI] [PubMed] [Google Scholar]
- Mukherjee A., Wong T. M., Templeton G., Buja L. M., Willerson J. T. Influence of volume dilution, lactate, phosphate, and calcium on mitochondrial functions. Am J Physiol. 1979 Aug;237(2):H224–H238. doi: 10.1152/ajpheart.1979.237.2.H224. [DOI] [PubMed] [Google Scholar]
- PAGE E. Cat heart muscle in vitro. III. The extracellular space. J Gen Physiol. 1962 Nov;46:201–213. doi: 10.1085/jgp.46.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter T., Heng M. K., Singh B. N., Ambler P., Nisbet H., Elliot R., Norris R. M. Failure of high doses of propranolol to reduce experimental myocardial ischemic damage. Circulation. 1978 Mar;57(3):534–540. doi: 10.1161/01.cir.57.3.534. [DOI] [PubMed] [Google Scholar]
- Pine M. B., Bing O. H., Brooks W. W., Abelmann W. H. Changes in in vitro myocardial hydration and performance in response to transient metabolic blockade in hypertonic, isotonic, and hypotonic media. Cardiovasc Res. 1978 Oct;12(10):569–577. doi: 10.1093/cvr/12.10.569. [DOI] [PubMed] [Google Scholar]
- Pine M. B., Bing O. H., Weintraub R., Abelmann W. H. Dissociation of cell volume regulation and sodium-potassium exchange pump activity in dog myocardium in vitro. J Mol Cell Cardiol. 1979 Jun;11(6):585–590. doi: 10.1016/0022-2828(79)90432-2. [DOI] [PubMed] [Google Scholar]
- Polimeni P. I., Al-Sadir J. Expansion of extracellular space in the nonischemic zone of the infarcted heart and concomitant changes in tissue electrolyte contents in the rat. Circ Res. 1975 Dec;37(6):725–732. doi: 10.1161/01.res.37.6.725. [DOI] [PubMed] [Google Scholar]
- Powell W. J., Jr, DiBona D. R., Flores J., Leaf A. The protective effect of hyperosmotic mannitol in myocardial ischemia and necrosis. Circulation. 1976 Oct;54(4):603–615. doi: 10.1161/01.cir.54.4.603. [DOI] [PubMed] [Google Scholar]
- Powell W. J., Wittenberg J., Miller S. W., Maturi R. A., Dinsmore R. E. Assessment of drug intervention on the ischemic myocardium: serial imaging and measurement with computerized tomography. Am J Cardiol. 1979 Jul;44(1):46–52. doi: 10.1016/0002-9149(79)90249-2. [DOI] [PubMed] [Google Scholar]
- Rasmussen M. M., Reimer K. A., Kloner R. A., Jennings R. B. Infarct size reduction by propranolol before and after coronary ligation in dogs. Circulation. 1977 Nov;56(5):794–798. doi: 10.1161/01.cir.56.5.794. [DOI] [PubMed] [Google Scholar]
- Smithen C., Christodoulou J., Killip T., Brachfeld N. Metabolic and hemodynamic consequences of mannitol following myocardial anoxia. Am J Physiol. 1975 Sep;229(3):847–852. doi: 10.1152/ajplegacy.1975.229.3.847. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A. Structure and function of intercellular junctions. Int Rev Cytol. 1974;39:191–283. doi: 10.1016/s0074-7696(08)60940-7. [DOI] [PubMed] [Google Scholar]
- Stephenson E. W. Functional extracellular space of smooth muscle in vitro. Am J Physiol. 1971 Jun;220(6):1728–1733. doi: 10.1152/ajplegacy.1971.220.6.1728. [DOI] [PubMed] [Google Scholar]
- Tarr M., Luckstead E. F., Jurewicz P. A., Haas H. G. Effect of propranolol on the fast inward sodium current in frog atrial muscle. J Pharmacol Exp Ther. 1973 Mar;184(3):599–610. [PubMed] [Google Scholar]
- Whalen D. A., Jr, Hamilton D. G., Ganote C. E., Jennings R. B. Effect of a transient period of ischemia on myocardial cells. I. Effects on cell volume regulation. Am J Pathol. 1974 Mar;74(3):381–397. [PMC free article] [PubMed] [Google Scholar]
- Wildenthal K., Dees J. H., Buja L. M. Cardiac lysosomal derangements in mouse heart after long-term exposure to nonmetabolizable sugars. Circ Res. 1977 Jan;40(1):26–35. doi: 10.1161/01.res.40.1.26. [DOI] [PubMed] [Google Scholar]
- Willerson J. T., Powell W. J., Jr, Guiney T. E., Stark J. J., Sanders C. A., Leaf A. Improvement in myocardial function and coronary blood flow in ischemic myocardium after mannitol. J Clin Invest. 1972 Dec;51(12):2989–2998. doi: 10.1172/JCI107126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerson J. T., Scales F., Mukherjee A., Platt M., Templeton G. H., Fink G. S., Buja L. M. Abnormal myocardial fluid retention as an early manifestation of ischemic injury. Am J Pathol. 1977 Apr;87(1):159–188. [PMC free article] [PubMed] [Google Scholar]
- Willerson J. T., Watson J. T., Hutton I., Fixler D. E., Curry G. C., Templeton G. H. The influence of hypertonic mannitol on regional myocardial blood flow during acute and chronic myocardial ischemia in anesthetized and awake intact dogs. J Clin Invest. 1975 May;55(5):892–902. doi: 10.1172/JCI108017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerson J. T., Watson J. T., Hutton I., Templeton G. H., Fixler D. E. Reduced myocardial reflow and increased coronary vascular resistance following prolonged myocardial ischemia in the dog. Circ Res. 1975 Jun;36(6):771–781. doi: 10.1161/01.res.36.6.771. [DOI] [PubMed] [Google Scholar]









