Abstract
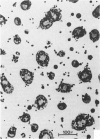
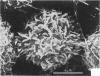
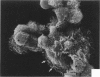
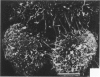
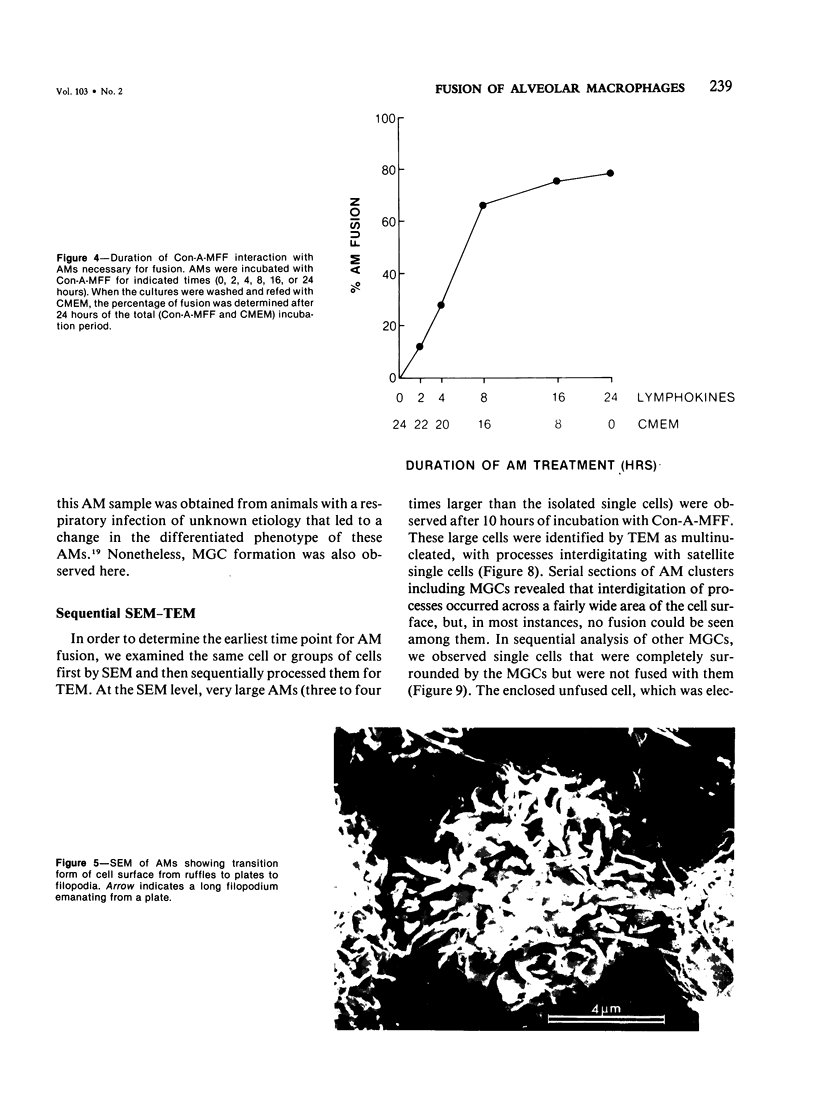
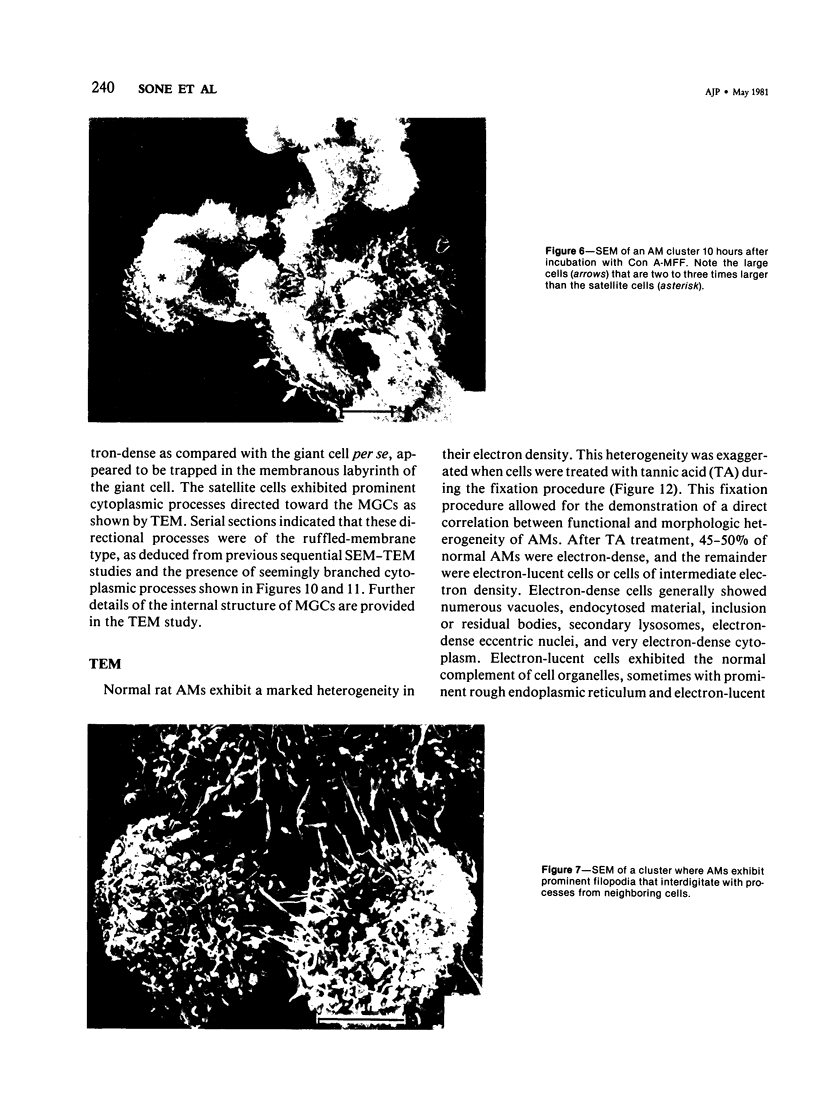
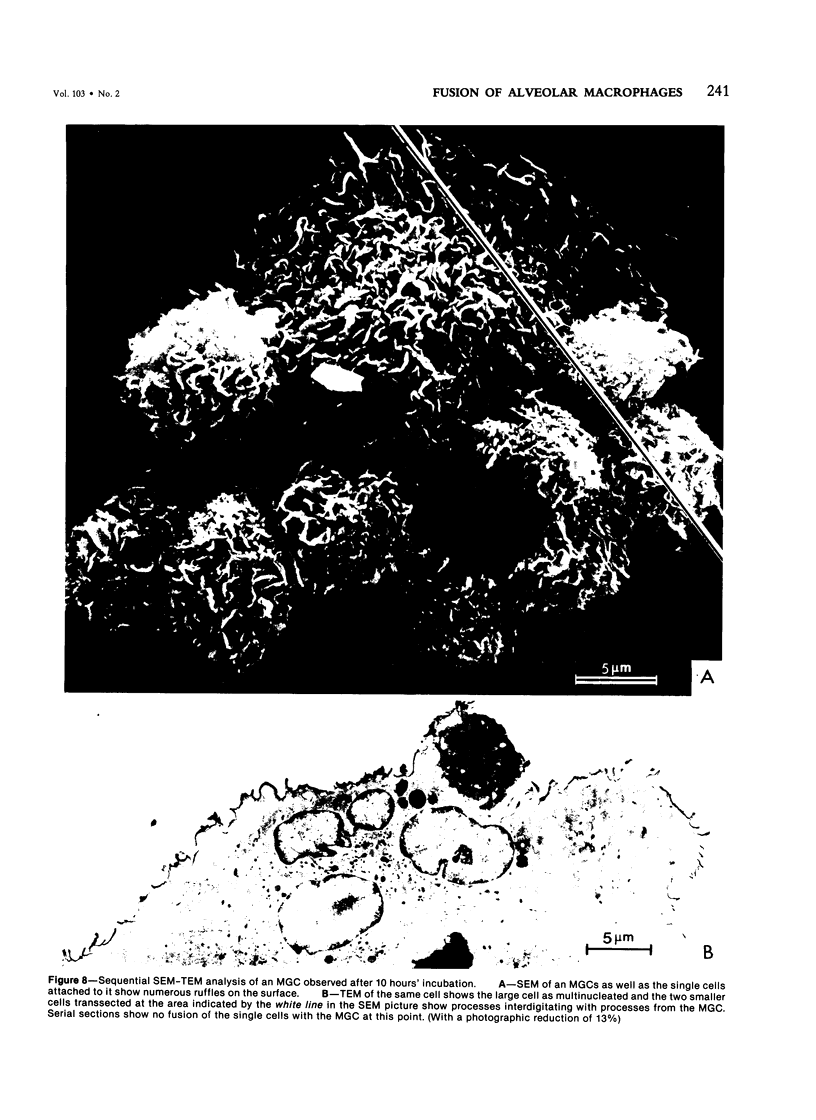
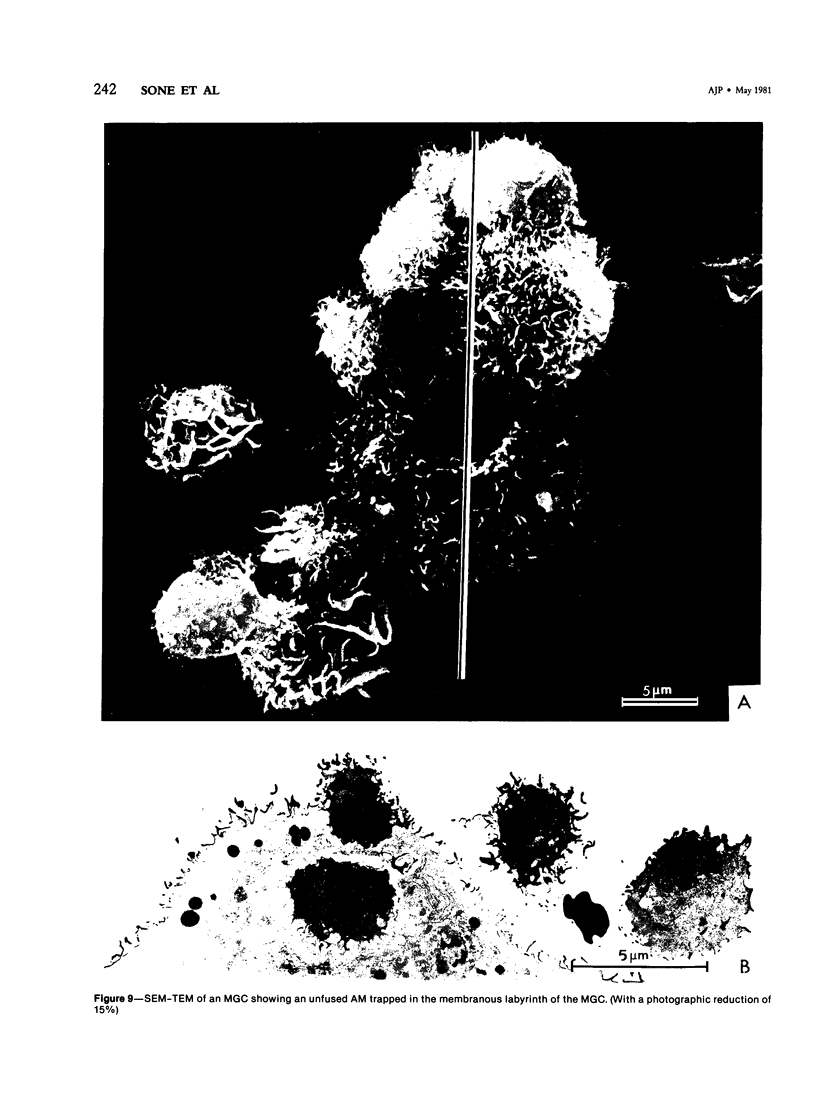
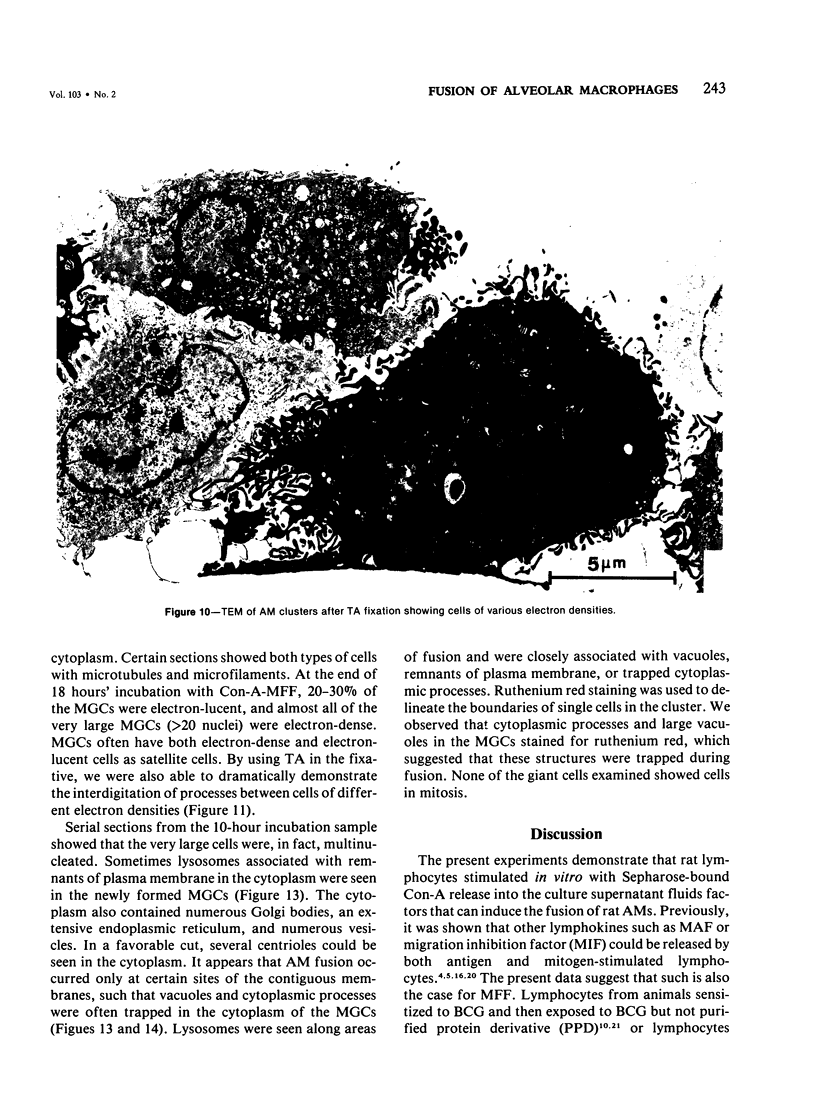
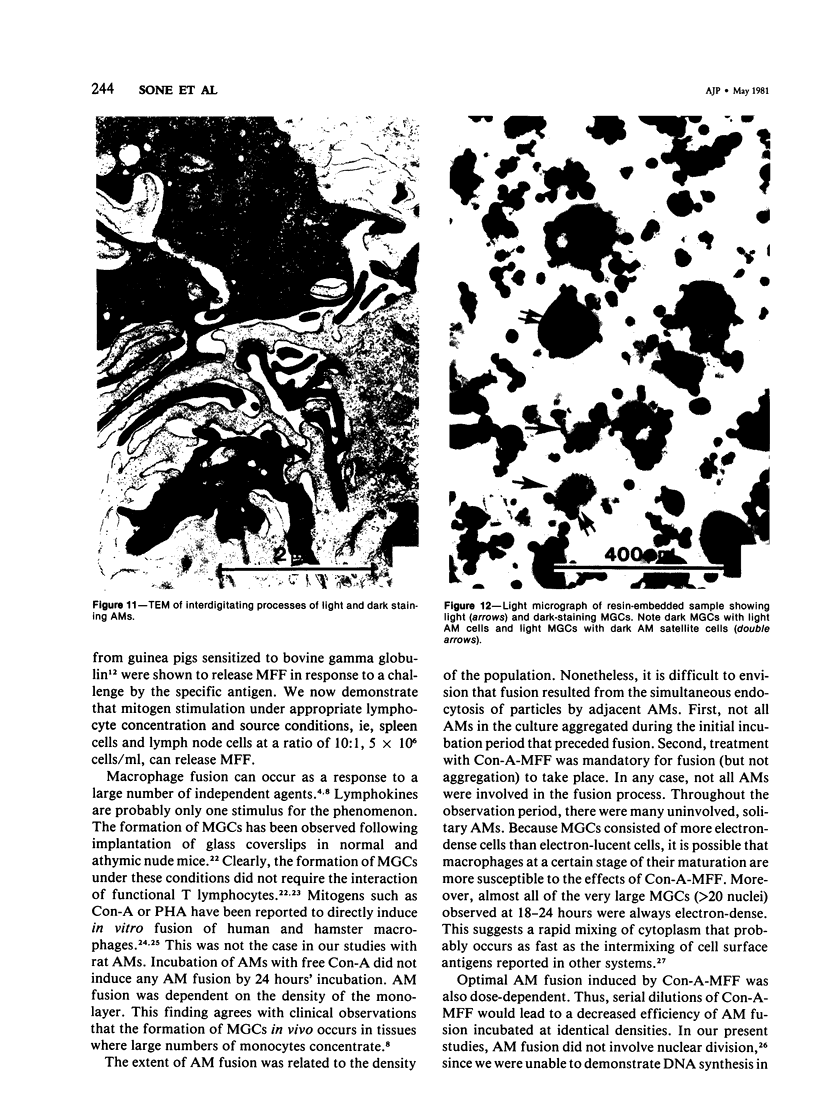
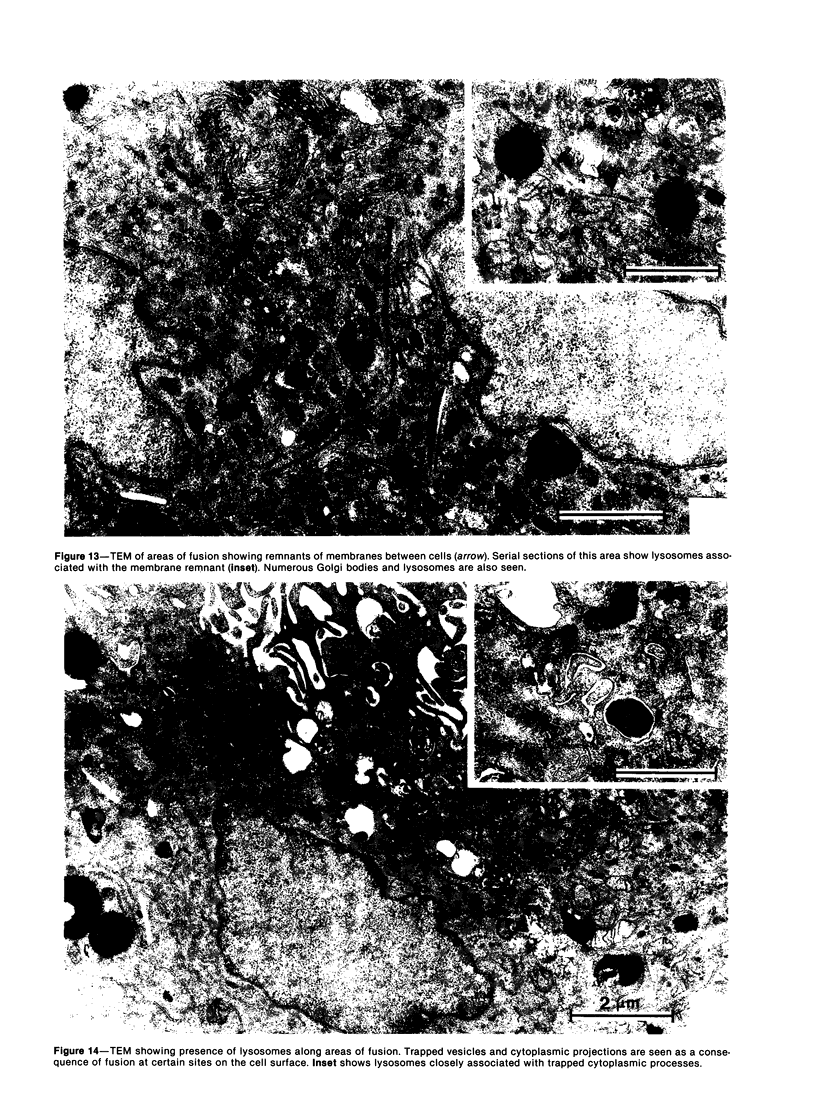
Treatment of F344 rat alveolar macrophages (AMs) in vitro with cell-free supernatant fluids obtained from concanavalin-A (Con A)-stimulated syngeneic lymphocytes induced extensive fusion. The lymphokine responsible for the fusion of AMs (but not other cells) is here referred to as AM fusion factor (Con-A-MFF). Fusion is dependent on the dose of Con-A-MFF and the population density of AM cultures and occurred 10 hours after Con-A-MFF was added to cultures of normal AMs. Con-A-MFF must interact with AMs for more than 8 hours before full expression of fusion is reached at 24 hours. Using a technique allowing for sequential scanning to transmission electron microscopy analysis of cells, the authors determined the relationship of the morphologic characteristics of the surface and the internal structure of cells fusing to form multinucleate giant cells (MGCs). The process of AM fusion begins with the aggregation of AMs, followed by interdigitation of cell processes. Serial sections of MGCs showed lysosomes associated with remnants of plasma membrane in the cytoplasm. The MGCs contained numerous organelles associated with increased secretory activity of cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. The granulomatous inflammatory response. A review. Am J Pathol. 1976 Jul;84(1):164–192. [PMC free article] [PubMed] [Google Scholar]
- Bucana C., Hoyer L. C., Hobbs B., Breesman S., McDaniel M., Hanna M. G., Jr Morphological evidence for the translocation of lysosomal organelles from cytotoxic macrophages into the cytoplasm of tumor target cells. Cancer Res. 1976 Dec;36(12):4444–4458. [PubMed] [Google Scholar]
- Chambers T. J. Fusion of hamster macrophages induced by lectins. J Pathol. 1977 Sep;123(1):53–61. doi: 10.1002/path.1711230107. [DOI] [PubMed] [Google Scholar]
- Chambers T. J. Multinucleate giant cells. J Pathol. 1978 Nov;126(3):125–148. doi: 10.1002/path.1711260302. [DOI] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Hibbs J. B., Jr Modulation of macrophage tumoricidal capability by components of normal serum: a central role for lipid. Science. 1977 Jul 15;197(4300):282–285. doi: 10.1126/science.195338. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Darnell J. H., Budmen M. B. In vitro activation of mouse macrophages by rat lymphocyte mediators. J Immunol. 1976 Aug;117(2):666–673. [PubMed] [Google Scholar]
- Fidler I. J., Darnell J. H., Budmen M. B. Tumoricidal properties of mouse macrophages activated with mediators from rat lymphocytes stimulated with concanavalin A. Cancer Res. 1976 Oct;36(10):3608–3615. [PubMed] [Google Scholar]
- Fidler I. J. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 1978 Sep;38(9):2651–2660. [PubMed] [Google Scholar]
- Frye L. D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970 Sep;7(2):319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- Galindo B., Lazdins J., Castillo R. Fusion of normal rabbit alveolar macrophages induced by supernatant fluids from BCG-sensitized lymph node cells after elicitation by antigen. Infect Immun. 1974 Feb;9(2):212–216. doi: 10.1128/iai.9.2.212-216.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo B., Rodríquez A. Affinity of macrophage fusion factor for macrophages. J Reticuloendothel Soc. 1979 Sep;26(3):221–227. [PubMed] [Google Scholar]
- Hadden J. W., Sadlik J. R., Hadden E. M. The induction of macrophage proliferation in vitro by a lymphocyte-produced factor. J Immunol. 1978 Jul;121(1):231–238. [PubMed] [Google Scholar]
- Hanna M. G., Jr, Snodgrass M. J., Zbar B., Rapp H. J. Histologic and ultrastructural studies of tumor regression in inbred guinea pigs after intralesional injection of Mycobacterium bovis (BCG). Natl Cancer Inst Monogr. 1973 Dec;39:71–85. [PubMed] [Google Scholar]
- Hibbs J. B., Jr Role of activated macrophages in nonspecific resistance to neoplasia. J Reticuloendothel Soc. 1976 Sep;20(3):223–231. [PubMed] [Google Scholar]
- Leake E. S., Wright M. J. Variations on the form of attachment of rabbit alveolar macrophages to various substrata as observed by scanning electron microscopy. J Reticuloendothel Soc. 1979 Apr;25(4):417–441. [PubMed] [Google Scholar]
- Machado E. A., Lair S. V. Giant multinucleate macrophages in methyl cellulose-stimulated athymic nude mice. J Reticuloendothel Soc. 1978 May;23(5):383–387. [PubMed] [Google Scholar]
- Papadimitriou J. M. The influence of the thymus on multinucleate giant cell formation. J Pathol. 1976 Mar;118(3):153–156. doi: 10.1002/path.1711180304. [DOI] [PubMed] [Google Scholar]
- Parks D. E., Weiser R. S. The role of phagocytosis and natural lymphokines in the fusion of alveolar macrophages to form Langhans giant cells. J Reticuloendothel Soc. 1975 Apr;17(4):219–228. [PubMed] [Google Scholar]
- Poste G. The tumoricidal properties of inflammatory tissue macrophages and multinucleate giant cells. Am J Pathol. 1979 Aug;96(2):595–610. [PMC free article] [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Macrophage activation for tumor cytotoxicity: induction of tumoricidal macrophages by supernatants of PPD-stimulated Bacillus Calmette-Guérin-immune spleen cell cultures. J Immunol. 1977 Sep;119(3):889–896. [PubMed] [Google Scholar]
- Smith C. W., Goldman A. S. Macrophages from human colostrum. Multinucleated giant cell formation by phytohemagglutinin and concanavalin A. Exp Cell Res. 1971 Jun;66(2):317–320. doi: 10.1016/0014-4827(71)90683-5. [DOI] [PubMed] [Google Scholar]
- Sone S., Poste G., Fidler I. J. Rat alveolar macrophages are susceptible to activation by free and liposome-encapsulated lymphokines. J Immunol. 1980 May;124(5):2197–2202. [PubMed] [Google Scholar]