Abstract
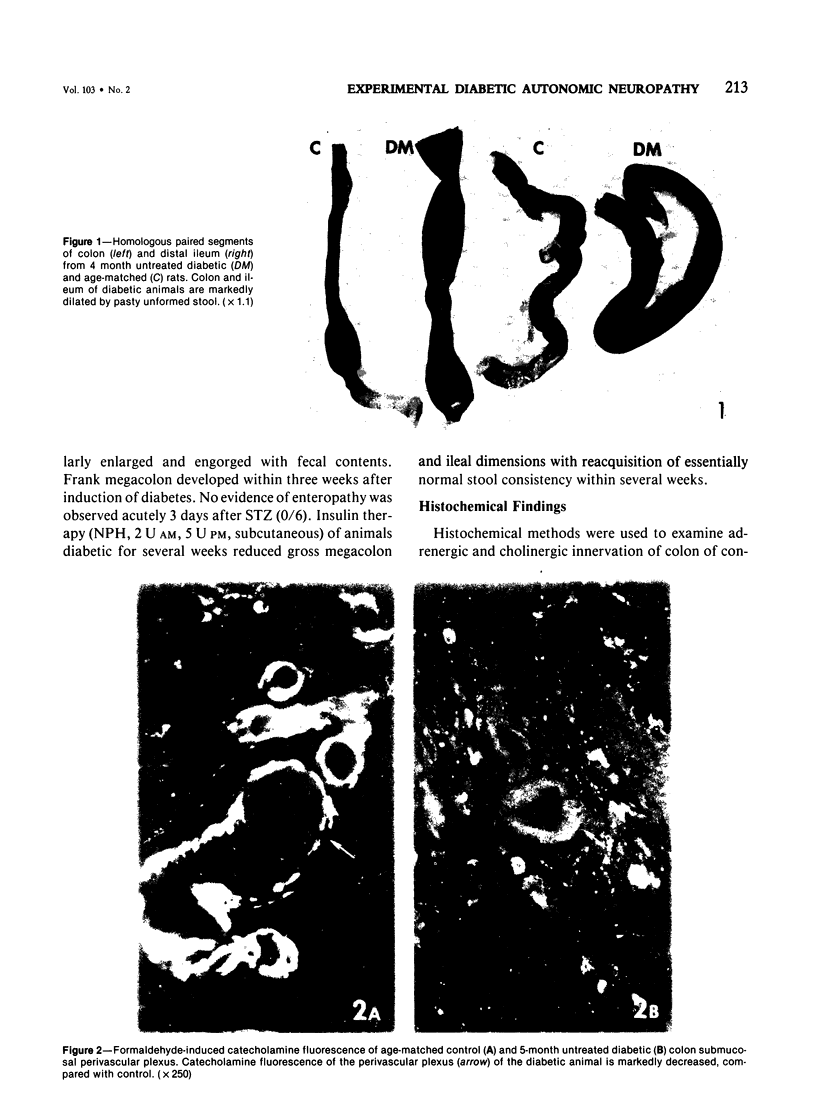
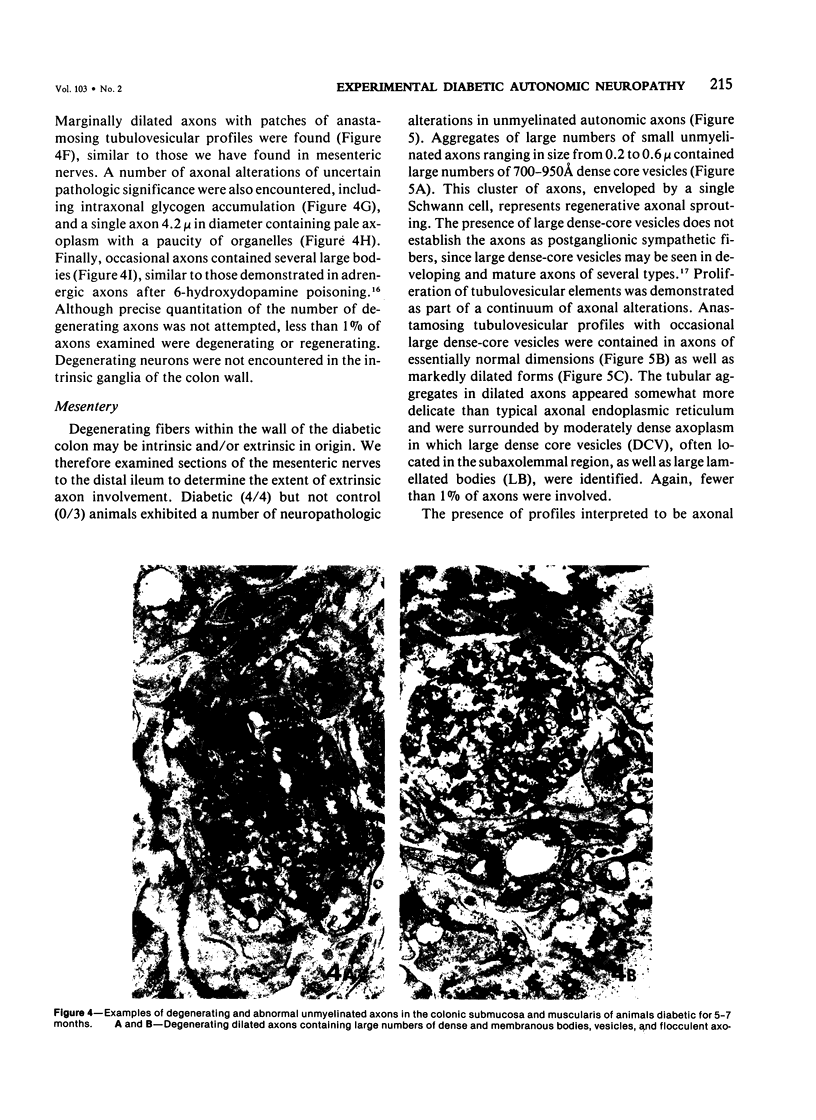
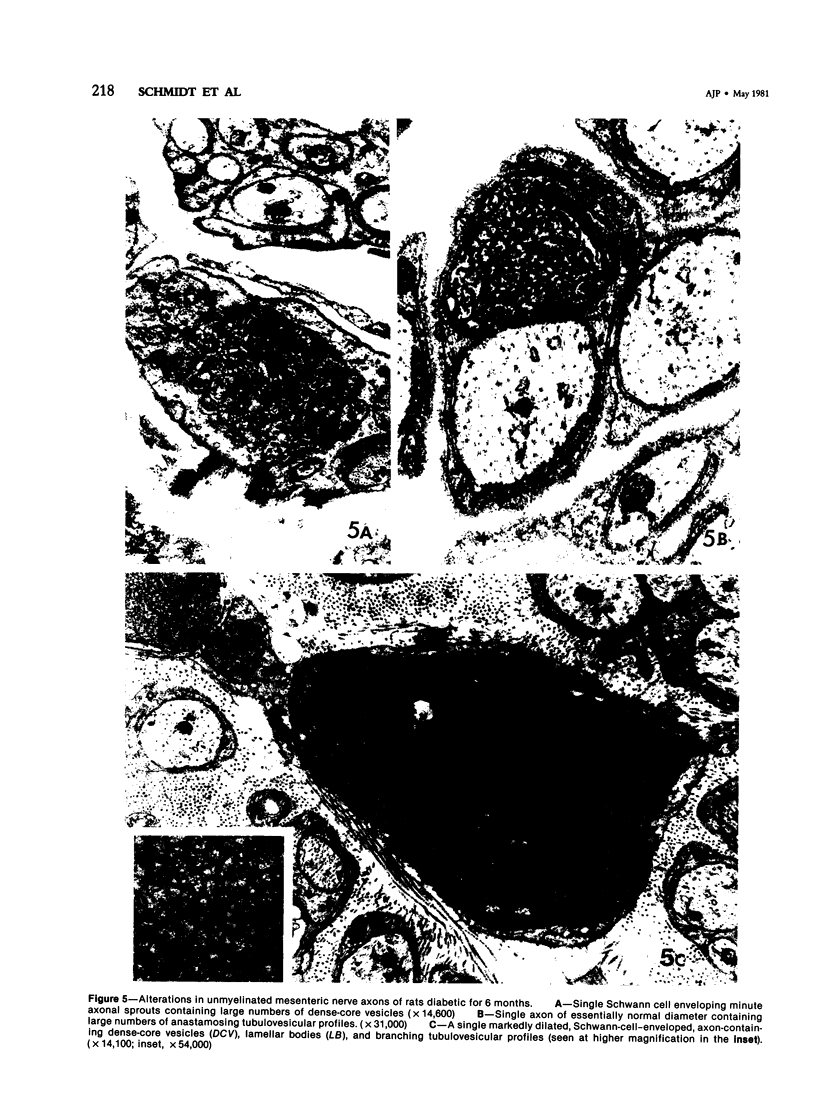
The regular occurrence of autonomic neuropathy, colonic dilatation, and loss of fecal consistency was investigated in streptozotocin-diabetic, age-matched control, and pancreatic-islet--transplanted rats using ultrastructural, histochemical, and biochemical methods. Degenerating unmyelinated axons were observed by electron microscopy in the colonic submucosa and muscularis, ileal mesentery, and splenic pedicle in 5--7 months diabetic animals; similar changes were not found in control rats or animals subjected to islet transplantation three weeks after induction of diabetes and sacrificed 4--6 months later (colon only). Regenerative changes, including axons with identifiable growth cones, were demonstrated in the mesenteric nerves of chronically diabetic animals. Formaldehyde-induced catecholamine fluorescence and cholinesterase histochemistry suggested deficiencies in colonic adrenergic and cholinergic innervation; histochemical findings in islet-transplanted animals were comparable to those of untreated control animals. Biochemical measurements of the adrenergic and cholinergic nervous system marker enzymes dopamine-beta-hydroxylase and choline acetyltransferase, respectively, in colon and spleen confirm a deficit in adrenergic (colon and spleen) and cholinergic (colon) innervation in chronically diabetic animals.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguayo A. J., Bray G. M., Terry L. C., Sweezey E. Three dimensional analysis of unmyelinated fibers in normal and pathologic autonomic nerves. J Neuropathol Exp Neurol. 1976 Mar;35(2):136–151. doi: 10.1097/00005072-197603000-00002. [DOI] [PubMed] [Google Scholar]
- Bisby M. A., Bulger V. T. Reversal of axonal transport at a nerve crush. J Neurochem. 1977 Aug;29(2):313–320. doi: 10.1111/j.1471-4159.1977.tb09624.x. [DOI] [PubMed] [Google Scholar]
- Bray G. M., Peyronnard J. M., Aguayo A. J. Reactions of unmyelinated nerve fibers to injury. An ultrastructural study. Brain Res. 1972 Jul 20;42(2):297–309. doi: 10.1016/0006-8993(72)90532-x. [DOI] [PubMed] [Google Scholar]
- Brown M. J., Martin J. R., Asbury A. K. Painful diabetic neuropathy. A morphometric study. Arch Neurol. 1976 Mar;33(3):164–171. doi: 10.1001/archneur.1976.00500030020004. [DOI] [PubMed] [Google Scholar]
- Bunge M. B. Fine structure of nerve fibers and growth cones of isolated sympathetic neurons in culture. J Cell Biol. 1973 Mar;56(3):713–735. doi: 10.1083/jcb.56.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLINS, GH, WEBSTERHDE F., VICTOR M. THE ULTRASTRUCTURE OF MYELIN AND AXONAL ALTERATIONS IN SCIATIC NERVES OF THIAMINE DEFICIENT AND CHRONICALLY STARVED RATS. Acta Neuropathol. 1964 May 5;3:511–521. doi: 10.1007/BF00688459. [DOI] [PubMed] [Google Scholar]
- Diani A. R., Grogan D. M., Yates M. E., Risinger D. L., Gerritsen G. C. Radiologic abnormalities and autonomic neuropathology in the digestive tract of the ketonuric diabetic Chinese hamster. Diabetologia. 1979 Jul;17(1):33–40. doi: 10.1007/BF01222975. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Hopkins A. P. Electron microscopic observations on degeneration and regeneration of unmyelinated fibres. Brain. 1972;95(2):233–234. doi: 10.1093/brain/95.2.223. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Johnson W. J., Lambert E. H., O'Brien P. C. Segmental demyelination secondary to axonal degeneration in uremic neuropathy. Mayo Clin Proc. 1971 Jun;46(6):400–431. [PubMed] [Google Scholar]
- Giachetti A. Axoplasmic transport of noradrenaline in the sciatic nerves of spontaneously diabetic mice. Diabetologia. 1979 Mar;16(3):191–194. doi: 10.1007/BF01219797. [DOI] [PubMed] [Google Scholar]
- Giachetti A. The functional state of sympathetic nerves in spontaneously diabetic mice. Diabetes. 1978 Oct;27(10):969–974. doi: 10.2337/diab.27.10.969. [DOI] [PubMed] [Google Scholar]
- Henry D. P., Johnson D. G., Starman B. J., Williams R. H. Kinetic characterization of rat serum dopamine-beta-hydroxylase using a simplified radioenzymatic assay. Life Sci. 1975 Oct 10;17(7):1179–1186. doi: 10.1016/0024-3205(75)90341-0. [DOI] [PubMed] [Google Scholar]
- Hildebrand J., Joffroy A., Graff G., Coërs C. Neuromuscular changes with alloxan hyperglycemia. Electrophysiological, biochemical, and histological study in rats. Arch Neurol. 1968 Jun;18(6):633–641. doi: 10.1001/archneur.1968.00470360055005. [DOI] [PubMed] [Google Scholar]
- Hosking D. J., Bennett T., Hampton J. R. Diabetic autonomic neuropathy. Diabetes. 1978 Oct;27(10):1043–1055. doi: 10.2337/diab.27.10.1043. [DOI] [PubMed] [Google Scholar]
- Iwayama T. Ultrastructural changes in the nerves innervating the cerebral artery after sympathectomy. Z Zellforsch Mikrosk Anat. 1970;109(4):465–480. doi: 10.1007/BF00343962. [DOI] [PubMed] [Google Scholar]
- Jakobsen J. Axonal dwindling in early experimental diabetes. I. A study of cross sectioned nerves. Diabetologia. 1976 Dec;12(6):539–546. doi: 10.1007/BF01220629. [DOI] [PubMed] [Google Scholar]
- Jakobsen J., Sidenius P. Decreased axonal flux of retrogradely transported glycoproteins in early experimental diabetes. J Neurochem. 1979 Nov;33(5):1055–1060. doi: 10.1111/j.1471-4159.1979.tb05241.x. [DOI] [PubMed] [Google Scholar]
- Jakobsen J., Sidenius P. Decreased axonal transport of structural proteins in streptozotocin diabetic rats. J Clin Invest. 1980 Aug;66(2):292–297. doi: 10.1172/JCI109856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. M., Jr, O'Brien F., Werbitt R. Modification and characterization of the permanent sympathectomy produced by the administration of guanethidine to newborn rats. Eur J Pharmacol. 1976 May;37(1):45–54. doi: 10.1016/0014-2999(76)90006-6. [DOI] [PubMed] [Google Scholar]
- Kapeller K., Mayor D. An electron microscopic study of the early changes proximal to a constriction in sympathetic nerves. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1026):39–51. doi: 10.1098/rspb.1969.0010. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Lampert P. W. A comparative electron microscopic study of reactive, degenerating, regenerating, and dystrophic axons. J Neuropathol Exp Neurol. 1967 Jul;26(3):345–368. doi: 10.1097/00005072-196707000-00001. [DOI] [PubMed] [Google Scholar]
- Lampert P. W., Schochet S. S., Jr Demyelination and remyelination in lead neuropathy. Electron microscopic studies. J Neuropathol Exp Neurol. 1968 Oct;27(4):527–545. [PubMed] [Google Scholar]
- Lentz T. L. Fine structure of nerves in the regenerating limb of the newt Triturus. Am J Anat. 1967 Nov;121(3):647–669. doi: 10.1002/aja.1001210312. [DOI] [PubMed] [Google Scholar]
- Monckton G., Pehowich E. Autonomic neuropathy in the streptozotocin diabetic rat. Can J Neurol Sci. 1980 May;7(2):135–142. doi: 10.1017/s0317167100023519. [DOI] [PubMed] [Google Scholar]
- Nelson J. S., Wakefield P. L. The cellular localization of catecholamines in frozen-dried cryostat sections of the brain and autonomic nervous system. J Neuropathol Exp Neurol. 1968 Apr;27(2):221–233. doi: 10.1097/00005072-196804000-00004. [DOI] [PubMed] [Google Scholar]
- Newson B., Ahlman H., Dahlström A., Das Gupta T. K., Nyhus L. M. On the innervation of the ileal mucosa in the rat--a synapse. Acta Physiol Scand. 1979 Mar;105(3):387–389. doi: 10.1111/j.1748-1716.1979.tb06357.x. [DOI] [PubMed] [Google Scholar]
- Ochoa J. Isoniazid neuropathy in man: quantitative electron microscope study. Brain. 1970;93(4):831–850. doi: 10.1093/brain/93.4.831. [DOI] [PubMed] [Google Scholar]
- Ochs S. Local supply of energy to the fast axoplasmic transport mechanism. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1279–1282. doi: 10.1073/pnas.68.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs S., Worth R. Batrachotoxin block of fast axoplasmic transport in mammalian nerve fibers. Science. 1975 Mar 21;187(4181):1087–1089. doi: 10.1126/science.46619. [DOI] [PubMed] [Google Scholar]
- Pawlik F., Bischoff A., Bitsch I. Peripheral nerve changes in thiamine deficiency and starvation. An electron microscopic study. Acta Neuropathol. 1977 Aug 31;39(3):211–218. doi: 10.1007/BF00691699. [DOI] [PubMed] [Google Scholar]
- Pleasure D. E., Mishler K. C., Engel W. K. Axonal transport of proteins in experimental neuropathies. Science. 1969 Oct 24;166(3904):524–525. doi: 10.1126/science.166.3904.524. [DOI] [PubMed] [Google Scholar]
- Powell H., Knox D., Lee S., Charters A. C., Orloff M., Garrett R., Lampert P. Alloxan diabetic neuropathy: electron microscopic studies. Neurology. 1977 Jan;27(1):60–66. doi: 10.1212/wnl.27.1.60. [DOI] [PubMed] [Google Scholar]
- Preston G. M. Peripheral neuropathy in the alloxan-diabetic rat. J Physiol. 1967 Apr;189(2):49P–50P. [PMC free article] [PubMed] [Google Scholar]
- Prineas J. The pathogenesis of dying-back polyneuropathies. II. An ultrastructural study of experimental acrylamide intoxication in the cat. J Neuropathol Exp Neurol. 1969 Oct;28(4):598–621. doi: 10.1097/00005072-196910000-00004. [DOI] [PubMed] [Google Scholar]
- Rees R. P., Bunge M. B., Bunge R. P. Morphological changes in the neuritic growth cone and target neuron during synaptic junction development in culture. J Cell Biol. 1976 Feb;68(2):240–263. doi: 10.1083/jcb.68.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. M., Sima A. A. Diabetic neuropathy in the mutant mouse [C57BL/ks(db/db)]: a morphometric study. Diabetes. 1980 Jan;29(1):60–67. doi: 10.2337/diab.29.1.60. [DOI] [PubMed] [Google Scholar]
- Schaumburg H. H., Wiśniewski H. M., Spencer P. S. Ultrastructural studies of the dying-back process. I. Peripheral nerve terminal and axon degeneration in systemic acrylamide intoxication. J Neuropathol Exp Neurol. 1974 Apr;33(2):260–284. doi: 10.1097/00005072-197404000-00006. [DOI] [PubMed] [Google Scholar]
- Schmidt R. E., Matschinsky F. M., Godfrey D. A., Williams A. D., McDougal D. B., Jr Fast and slow axoplasmic flow in sciatic nerve of diabetic rats. Diabetes. 1975 Dec;24(12):1081–1085. doi: 10.2337/diab.24.12.1081. [DOI] [PubMed] [Google Scholar]
- Schmidt R. E., Yu M. J., McDougal D. B., Jr Turnaround of axoplasmic transport of selected particle-specific enzymes at an injury in control and diisopropylphosphorofluoridate-treated rats. J Neurochem. 1980 Sep;35(3):641–652. doi: 10.1111/j.1471-4159.1980.tb03703.x. [DOI] [PubMed] [Google Scholar]
- Schrier B. K., Shuster L. A simplified radiochemical assay for choline acetyltransferase. J Neurochem. 1967 Oct;14(10):977–985. doi: 10.1111/j.1471-4159.1967.tb09509.x. [DOI] [PubMed] [Google Scholar]
- Sharma A. K., Thomas P. K. Peripheral nerve structure and function in experimental diabetes. J Neurol Sci. 1974 Sep;23(1):1–15. doi: 10.1016/0022-510x(74)90136-1. [DOI] [PubMed] [Google Scholar]
- Spencer P. S., Thomas P. K. Ultrastructural studies of the dying-back process. II. The sequestration and removal by Schwann cells and oligodendrocytes of organelles from normal and diseases axons. J Neurocytol. 1974 Dec;3(6):763–783. doi: 10.1007/BF01097197. [DOI] [PubMed] [Google Scholar]
- Tranzer J. P., Thoenen H. An electron microscopic study of selective, acute degeneration of sympathetic nerve terminals after administration of 6-hydroxydopamine. Experientia. 1968 Feb 15;24(2):155–156. doi: 10.1007/BF02146956. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Ishikawa H. The movement of membranous organelles in axons. Electron microscopic identification of anterogradely and retrogradely transported organelles. J Cell Biol. 1980 Mar;84(3):513–530. doi: 10.1083/jcb.84.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]