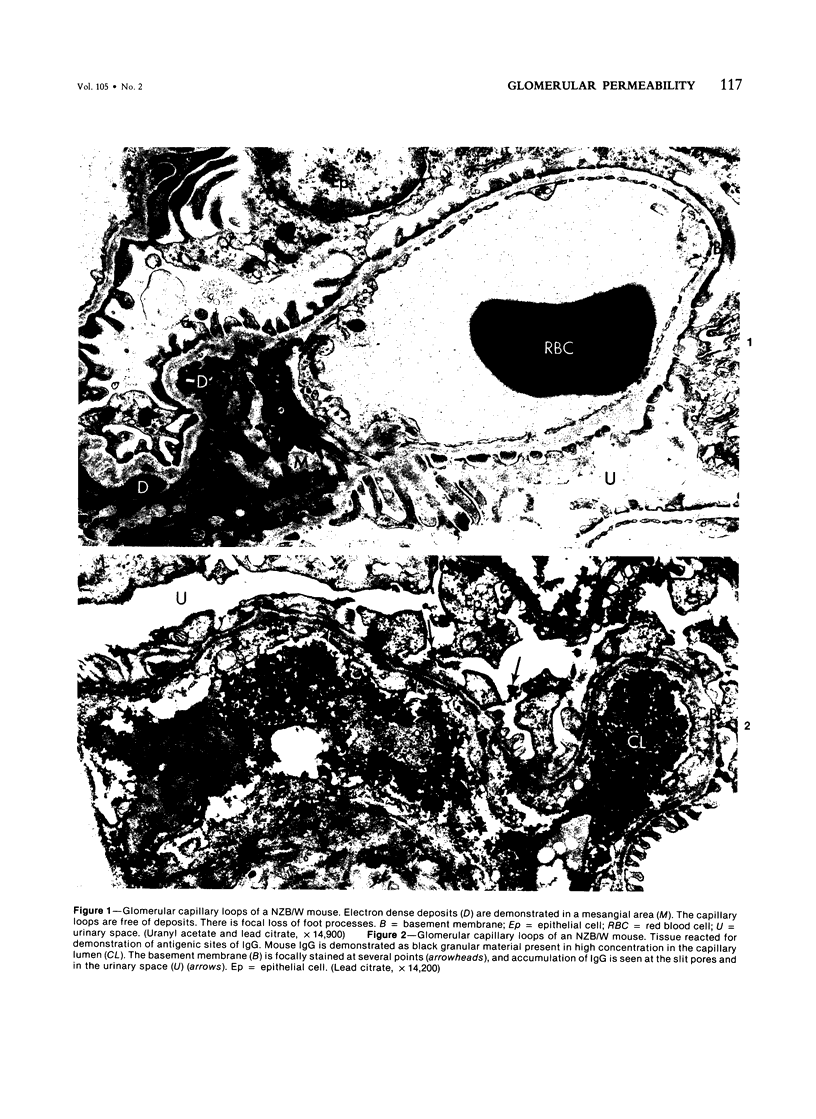
Abstract
To verify whether an increase in glomerular permeability precedes the deposition of immune complexes in the capillary wall, the following were studied in mice with lupus nephritis: 1) urinary proteins; 2) glomerular transfer of IgG, albumin, and anionic ferritin (AF) (isoelectric point, 4.2-4.6); and 3) anionic groups of the capillary wall as detected by binding of cationized ferritin (CF) (isoelectric point, 7.5-8.6). The glomeruli were investigated by immunofluorescence, immunoelectron, and transmission electron-microscopic studies. Urinary proteins were quantitated and characterized by electrophoresis. When mice were 5 months of age, (the time at which pathologic proteinuria was first detected), immune deposits were few and were confined to the mesangium. Although AF molecules were largely retained at the level of the lamina rara interna, focal leakage of albumin and IgG was detected across capillary walls that were not found to contain immune deposits. Focal and irregular loss of anionic groups, reflected by decreased binding of CF molecules, occurred in the laminae rarae of the basement membrane. Foot processes of glomeruli incubated with CF showed no loss of polyanion. We conclude that the increase in glomerular permeability that precedes deposition of immune complexes is due, in part, to focal loss of anionic groups of the basement membrane.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- BENACERRAF B., McCLUSKEY R. T., PATRAS D. Localization of colloidal substances in vascular endothelium: a mechanism of tissue damage. I. Factors causing the pathologic deposition of colloidal carbon. Am J Pathol. 1959 Jan-Feb;35(1):75–91. [PMC free article] [PubMed] [Google Scholar]
- Benveniste J., Egido J., Gutierrez-Millet V. Evidence for the involvement of IgE-basophil system in acute serum sickness. Clin Exp Immunol. 1976 Dec;26(3):449–456. [PMC free article] [PubMed] [Google Scholar]
- COCHRANE C. G. STUDIES ON THE LOCALIZATION OF CIRCULATING ANTIGEN-ANTIBODY COMPLEXES AND OTHER MACROMOLECULES IN VESSELS. II. PATHOGENETIC AND PHARMACODYNAMIC STUDIES. J Exp Med. 1963 Oct 1;118:503–513. doi: 10.1084/jem.118.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo T., Graves K., Granholm N. A. Endothelial and perivascular anionic sites during immediate transient vascular leakage. Virchows Arch A Pathol Anat Histol. 1980;388(1):1–12. doi: 10.1007/BF00430673. [DOI] [PubMed] [Google Scholar]
- Couser W. G., Jermanovich N. B., Belok S., Stilmant M. M., Hoyer J. R. Effect of aminonucleoside nephrosis on immune complex localization in autologous immune complex nephropathy in rats. J Clin Invest. 1978 Mar;61(3):561–572. doi: 10.1172/JCI108967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. K., Cavallo T. Membranoproliferative glomerulonephritis. Localization of early components of complement in glomerular deposits. Am J Pathol. 1976 Aug;84(2):283–298. [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Howie J. B., Helyer B. J. The immunology and pathology of NZB mice. Adv Immunol. 1968;9:215–266. doi: 10.1016/s0065-2776(08)60444-7. [DOI] [PubMed] [Google Scholar]
- Kelley V. E., Cavallo T. Glomerular permeability. Ultrastructural studies in New Zealand black/white mice using polyanionic ferritin as a molecular probe. Lab Invest. 1977 Sep;37(3):265–275. [PubMed] [Google Scholar]
- Kelley V. E., Cavallo T. Glomerular permeability: focal loss of anionic sites in glomeruli of proteinuric mice with lupus nephritis. Lab Invest. 1980 Jan;42(1):59–64. [PubMed] [Google Scholar]
- Kelley V. E., Cavallo T. Glomerular permeability: transfer of native ferritin in glomeruli with decreased anionic sites. Lab Invest. 1978 Dec;39(6):547–553. [PubMed] [Google Scholar]
- Kniker W. T., Cochrane C. G. The localization of circulating immune complexes in experimental serum sickness. The role of vasoactive amines and hydrodynamic forces. J Exp Med. 1968 Jan 1;127(1):119–136. doi: 10.1084/jem.127.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama A., Niwa Y., Shigematsu H., Taniguchi M., Tada T. Studies on passive serum sickness. II. Factors determining the localization of antigen-antibody complexes in the murine renal glomerulus. Lab Invest. 1978 Mar;38(3):253–262. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambert P. H., Dixon F. J. Pathogenesis of the glomerulonephritis of NZB/W mice. J Exp Med. 1968 Mar 1;127(3):507–522. doi: 10.1084/jem.127.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B. Increased permeability to albumin induced with protamine in modified gelatine membranes. Nature. 1967 Aug 5;215(5101):641–642. doi: 10.1038/215641a0. [DOI] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G., Shea S. M., Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol. 1969 Sep;42(3):647–672. doi: 10.1083/jcb.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Neuhaus O. W., Flory W. The effect of dietary protein on the excretion of alpha2u, the sex-dependent protein of the adult male rat. Biochim Biophys Acta. 1975 Nov 10;411(1):74–86. doi: 10.1016/0304-4165(75)90286-x. [DOI] [PubMed] [Google Scholar]
- Olivetti G., Kithier K., Giacomelli F., Wiener J. Glomerular permeability to endogenous proteins in the rat: effects of acute hypertension. Lab Invest. 1981 Feb;44(2):127–137. [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parbtani A., Cameron J. S. Platelet and plasma serotonin concentration in glomerulonephritis I. Thromb Res. 1979;15(1-2):109–125. doi: 10.1016/0049-3848(79)90057-4. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler M. W., Venkatachalam M. A., Cotran R. S. Glomerular epithelium: structural alterations induced by polycations. Science. 1975 Aug 1;189(4200):390–393. doi: 10.1126/science.1145209. [DOI] [PubMed] [Google Scholar]
- Steward M. W., Hay F. C. Changes in immunoglobulin class and subclass of anti-DNA antibodies with increasing age in N/ZBW F1 hybrid mice. Clin Exp Immunol. 1976 Nov;26(2):363–370. [PMC free article] [PubMed] [Google Scholar]
- Steward M. W., Katz F. E., West N. J. The role of low affinity antibody in immune complex disease. The quantity of anti-DNA antibodies in NZB/W F1 hybrid mice. Clin Exp Immunol. 1975 Jul;21(1):121–130. [PMC free article] [PubMed] [Google Scholar]