Abstract
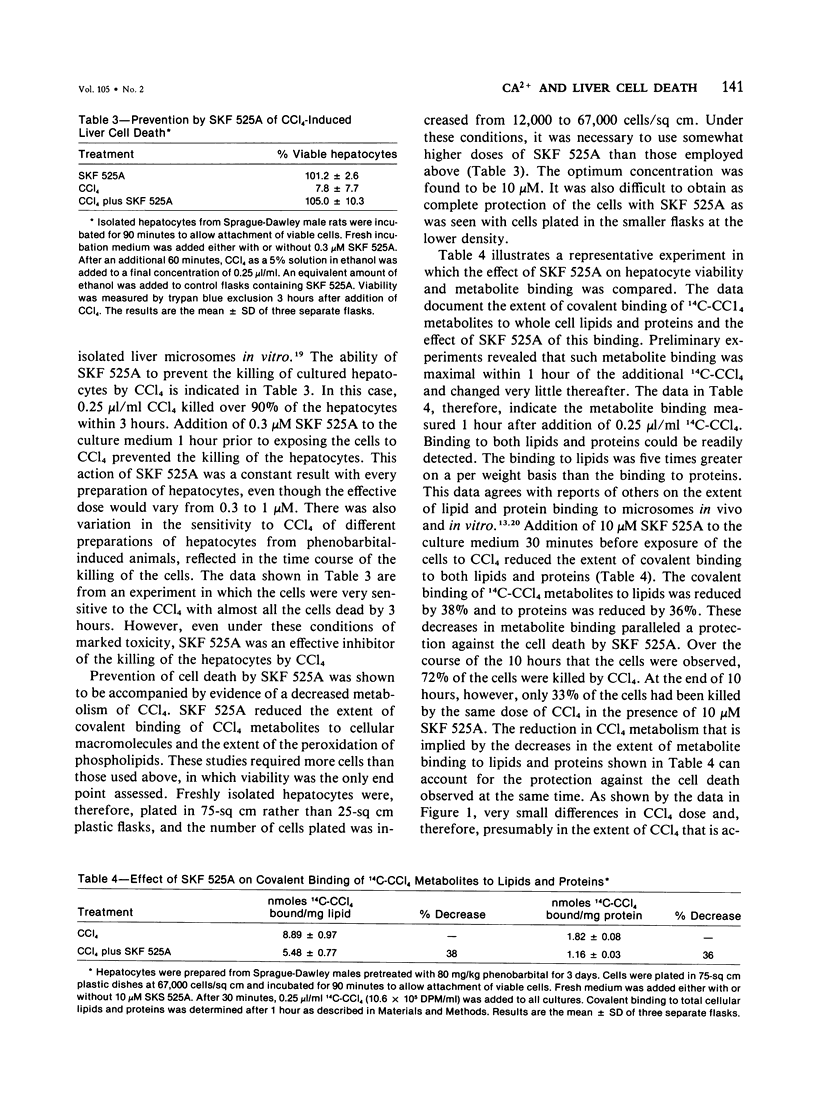
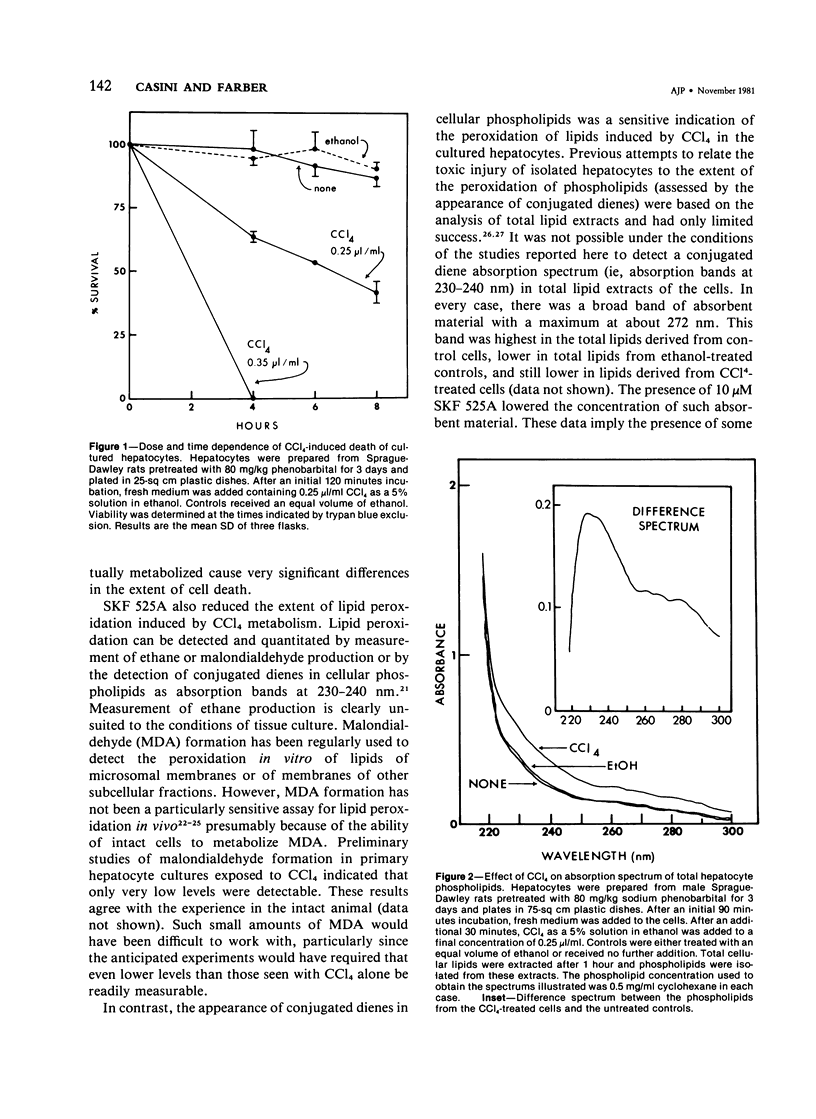
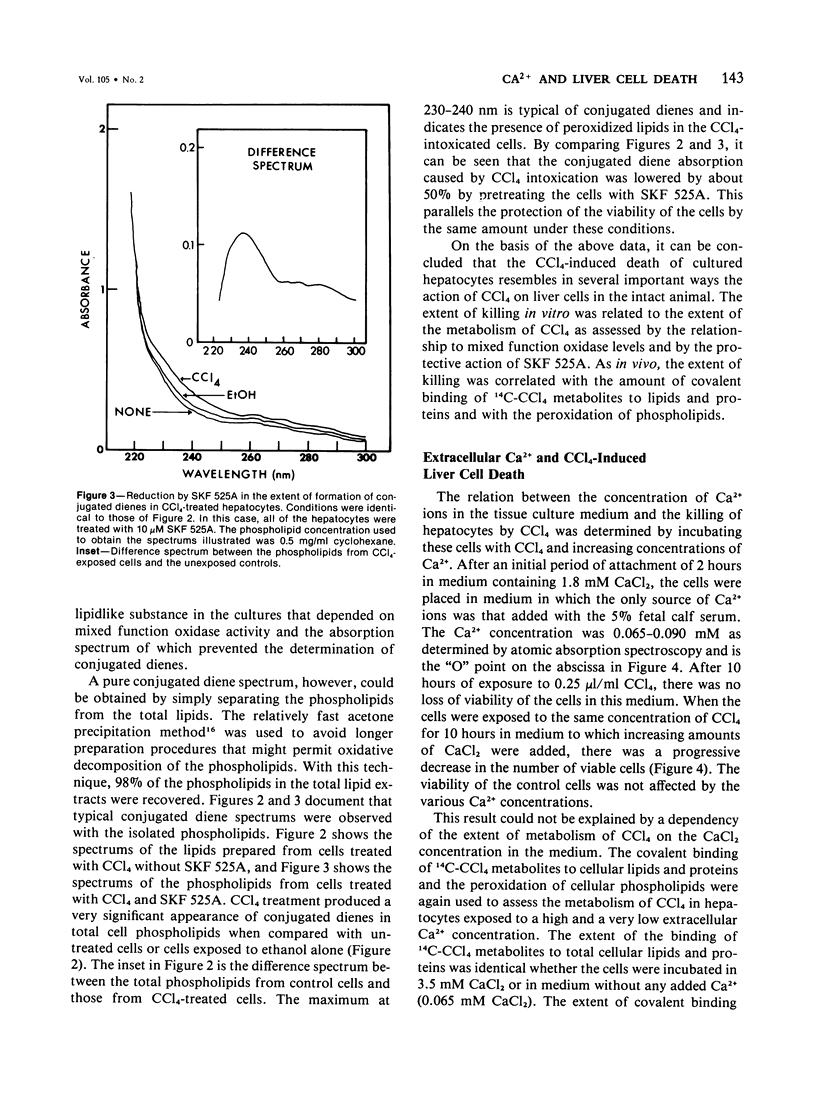
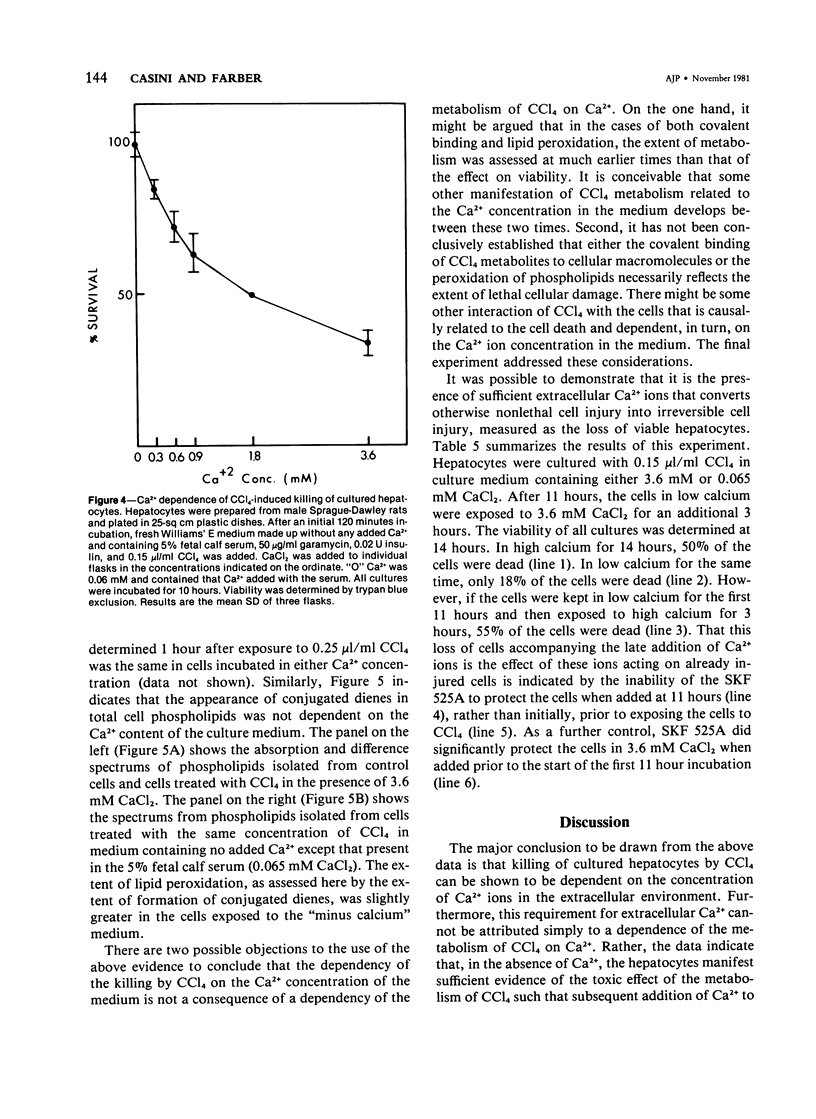
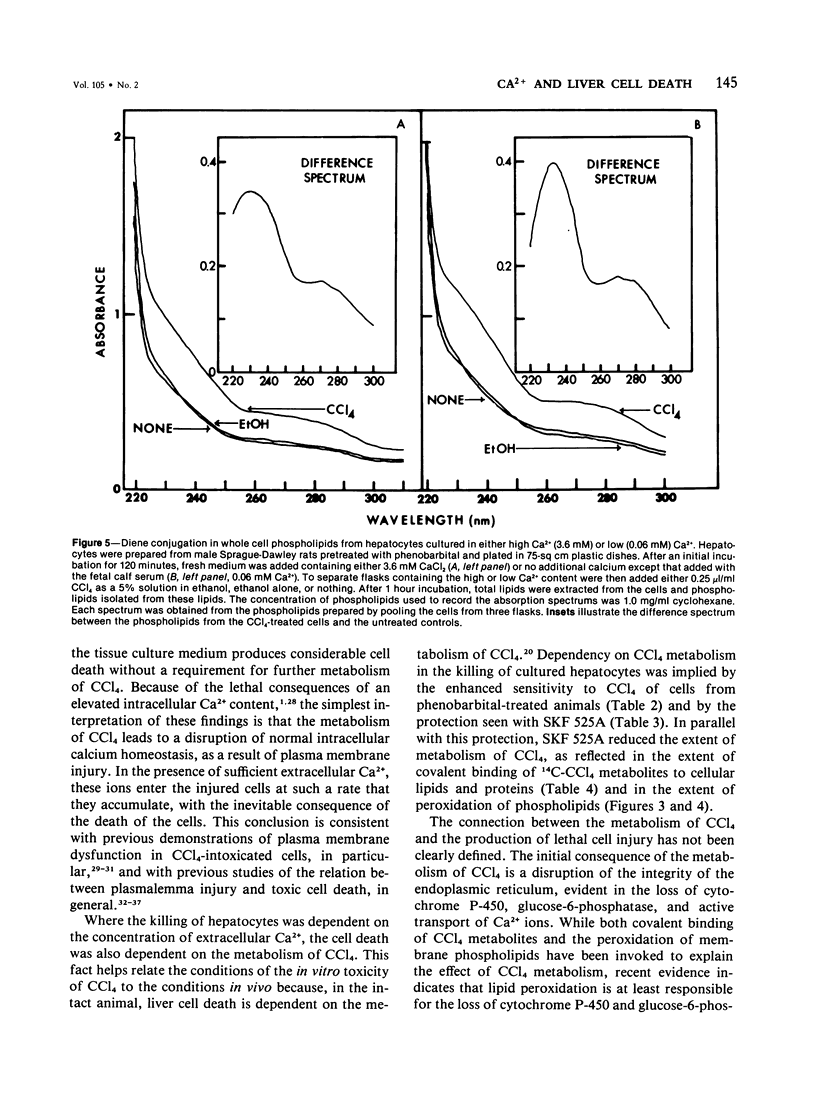
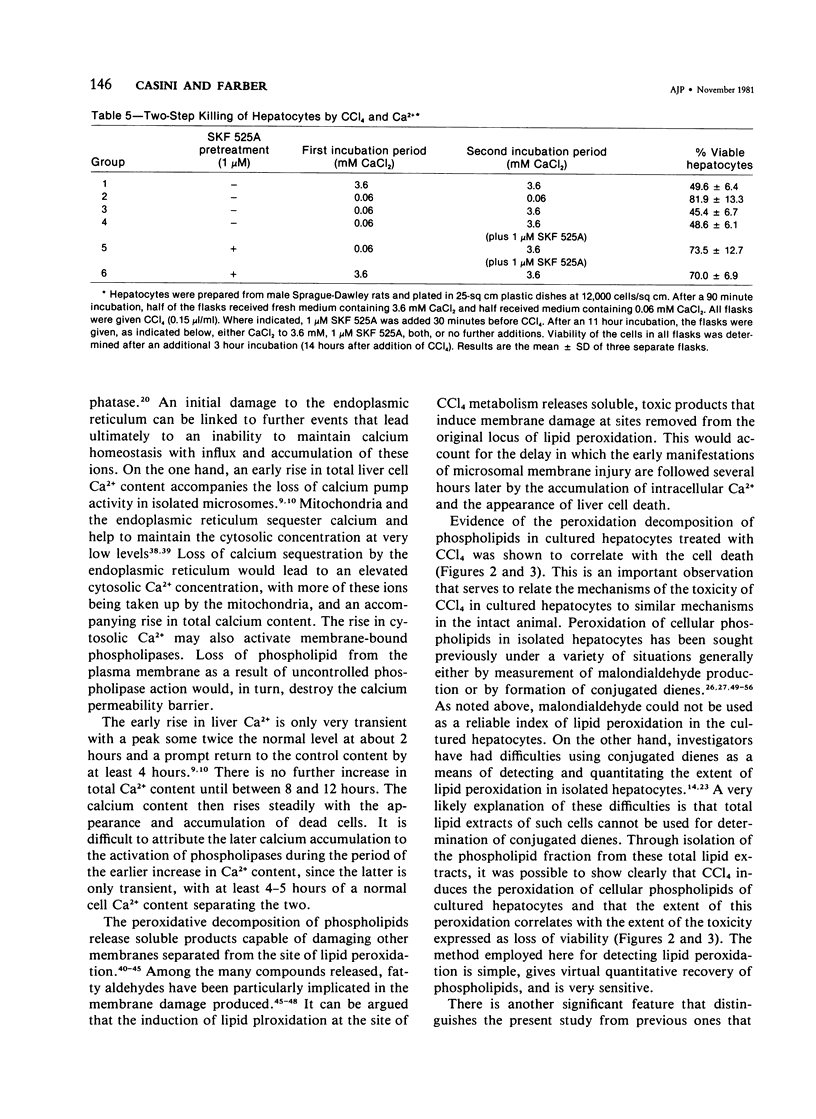
The role of extracellular Ca2+ ions in the killing of liver cells by CCl4 was studied in primary cultures of rat hepatocytes. The dependence of in vitro cell killing on the metabolism of CCl4 was first examined in order to document the similarity between the action of CCl4 on cultured hepatocytes and the action of CCl4 on liver cells in the intact animal. Cells prepared from male rats pretreated with phenobarbital were more sensitive to CCl4 than cells prepared from either male or female rats. The killing of hepatocytes by CCl4 was prevented by addition of SKF 525A to the culture medium. This protection was accompanied by evidence of decreased CCl4 metabolism as assessed by the extent of covalent binding of 14C-CCl4 metabolites to total cellular lipids and proteins, and by the extent of formation of conjugated dienes accompanying the peroxidation of phospholipids isolated from total cell lipids. The extent of killing of the hepatocytes by CCl4 was dependent on the Ca2+ concentration in the tissue culture medium. Total Ca2+ concentrations lower than 0.10 mM were not associated with any CCl4-induced cell death, and the number of dead cells increased with increasing Ca2+ from 0.3 to 3.6 mM. This dependency on extracellular Ca2+ was not due to dependency of the extent of metabolism of CCl4 on Ca2+. The Ca2+ concentration in the medium had no effect on the extent of covalent binding of metabolites of CCl4 to lipids and to proteins and on the extent of peroxidation of phospholipids as shown by the formation of conjugated dienes. In addition, hepatocytes incubated in low Ca2+ with CCl4 developed further evidence of cell injury, as indicated by the killing of these cells following the addition of high Ca2+ concentrations under conditions prohibiting any further metabolism of the CCl4. The results of this study indicate that it is the presence of extracellular Ca2+ that converts initially nonlethal cell injury into irreversible cell injury in CCl4-treated cells. This action of Ca2+ most likely represents an influx into the cell across an injured permeability barrier at the plasma membrane, in accord with the accumulation of large quantities of Ca2+ in CCl4-intoxicated liver cells in the intact animal. The relation between this alteration in Ca2+ homeostasis and the metabolism of CCl4 is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht D. K., Kappus H., Remmer H. Lipid peroxidation and cell damage in isolated hepatocytes due to bromotrichloromethane. Toxicol Appl Pharmacol. 1978 Nov;46(2):499–505. doi: 10.1016/0041-008x(78)90095-9. [DOI] [PubMed] [Google Scholar]
- BORGSTROM B. Investigation on lipid separation methods. Separation of phospholipids from neutral fat and fatty acids. Acta Physiol Scand. 1952 Jun 6;25(2-3):101–110. doi: 10.1111/j.1748-1716.1952.tb00862.x. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Casini A. F., Ferrali M., Comporti M. Effects of diffusible products of peroxidation of rat liver microsomal lipids. Biochem J. 1979 May 15;180(2):303–312. doi: 10.1042/bj1800303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti A., Casini A. F., Ferrali M., Comporti M. Extraction and partial characterization of dialysable products originating from the peroxidation of liver microsomal lipids and inhibiting microsomal glucose 6-phosphatase activity. Biochem Pharmacol. 1979 Oct 1;28(19):2909–2918. doi: 10.1016/0006-2952(79)90585-9. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Casini A. F., Ferrali M. Red cell lysis coupled to the peroxidation of liver microsomal lipids. Compartmentalization of the hemolytic system. Res Commun Chem Pathol Pharmacol. 1977 Jul;17(3):519–528. [PubMed] [Google Scholar]
- Benedetti A., Comporti M., Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim Biophys Acta. 1980 Nov 7;620(2):281–296. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Ferrali M., Casini A. F., Pieri S., Comporti M. Foot-edema induced by carbonyl compounds originating from the peroxidation of liver microsomal lipids. Biochem Pharmacol. 1980 Jan 1;29(1):121–124. doi: 10.1016/0006-2952(80)90256-7. [DOI] [PubMed] [Google Scholar]
- Casini A. F., Benedetti A., Ferrali M., Comporti M. Binding of products originating from the peroxidation of liver microsomal lipids to the non-lipid constituents of the microsomal membrane. Chem Biol Interact. 1979 May;25(2-3):211–227. doi: 10.1016/0009-2797(79)90047-4. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Abrams J., Pfau R. G., Farber J. L. Prevention by chlorpromazine of ischemic liver cell death. Am J Pathol. 1977 Sep;88(3):539–557. [PMC free article] [PubMed] [Google Scholar]
- Durant S., Homo F., Duval D. Calcium and A23187-induced cytolysis of mouse thymocytes. Biochem Biophys Res Commun. 1980 Mar 28;93(2):385–391. doi: 10.1016/0006-291x(80)91088-8. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Farber J. L., Chien K. R., Mittnacht S., Jr Myocardial ischemia: the pathogenesis of irreversible cell injury in ischemia. Am J Pathol. 1981 Feb;102(2):271–281. [PMC free article] [PubMed] [Google Scholar]
- Glende E. A., Jr, Hruszkewycz A. M., Recknagel R. O. Critical role of lipid peroxidation in carbon tetrachloride-induced loss of aminopyrine demethylase, cytochrome P-450 and glucose 6-phosphatase. Biochem Pharmacol. 1976 Oct 1;25(19):2163–2170. doi: 10.1016/0006-2952(76)90128-3. [DOI] [PubMed] [Google Scholar]
- Gravela E., Albano E., Dianzani M. U., Poli G., Slater T. F. Effects of carbon tetrachloride on isolated rat hepatocytes. Inhibition of protein and lipoprotein secretion. Biochem J. 1979 Feb 15;178(2):509–512. doi: 10.1042/bj1780509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLTKAMP D. E., HILL R. M. Comparison of malonate and malondialdehyde in vitro oxygen uptake studies. Arch Biochem Biophys. 1951 Nov;34(1):216–218. doi: 10.1016/s0003-9861(51)80027-4. [DOI] [PubMed] [Google Scholar]
- Horton A. A. Mitochondrial metabolism of aldehydes. Biochem J. 1970 Feb;116(4):19P–20P. doi: 10.1042/bj1160019pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Högberg J., Moldéus P., Arborgh B., O'Brien P. J., Orrenius S. The consequences of lipid peroxidation in isolated hepatocytes. Eur J Biochem. 1975 Nov 15;59(2):457–462. doi: 10.1111/j.1432-1033.1975.tb02474.x. [DOI] [PubMed] [Google Scholar]
- Högberg J., Orrenius S., Larson R. E. Lipid peroxidation in isolated hepatocytes. Eur J Biochem. 1975 Jan 15;50(3):595–602. doi: 10.1111/j.1432-1033.1975.tb09900.x. [DOI] [PubMed] [Google Scholar]
- Högberg J., Orrenius S., O'Brien P. J. Further studies on lipid-peroxide formation in isolated hepatocytes. Eur J Biochem. 1975 Nov 15;59(2):449–455. doi: 10.1111/j.1432-1033.1975.tb02473.x. [DOI] [PubMed] [Google Scholar]
- Izutsu K. T., Smuckler E. A. Effects of carbon tetrachloride on rat liver plasmalemmal calcium adenosine triphosphatase. Am J Pathol. 1978 Jan;90(1):145–158. [PMC free article] [PubMed] [Google Scholar]
- Kamath S. A., Rubin E. Effects of carbon tetrachloride and phenobarbital on plasma membranes. Enzymes and phospholipid transfer. Lab Invest. 1974 Apr;30(4):494–499. [PubMed] [Google Scholar]
- Kane A. B., Stanton R. P., Raymond E. G., Dobson M. E., Knafelc M. E., Farber J. L. Dissociation of intracellular lysosomal rupture from the cell death caused by silica. J Cell Biol. 1980 Dec;87(3 Pt 1):643–651. doi: 10.1083/jcb.87.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane A. B., Young E. E., Schanne F. A., Farber J. L. Calcium dependence of phalloidin-induced liver cell death. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1177–1180. doi: 10.1073/pnas.77.2.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiho K. U., Shelburne J. D., Trump B. F. Observations on cell volume, ultrastructure, mitochondrial conformation and vital-dye uptake in Ehrlich ascites tumor cells. Effects of inhibiting energy production and function of the plasma membrane. Am J Pathol. 1971 Oct;65(1):203–230. [PMC free article] [PubMed] [Google Scholar]
- Laiho K. U., Trump B. F. Relationship of ionic, water, and cell volume changes in cellular injury of Ehrlich ascites tumor cells. Lab Invest. 1974 Sep;31(3):207–215. [PubMed] [Google Scholar]
- Lindstrom T. D., Anders M. W., Remmer H. Effect of phenobarbital and diethyl maleate on carbon tetrachloride toxicity in isolated rat hepatocytes. Exp Mol Pathol. 1978 Feb;28(1):48–57. doi: 10.1016/0014-4800(78)90063-1. [DOI] [PubMed] [Google Scholar]
- Mittnacht S., Jr, Sherman S. C., Farber J. L. Reversal of ischemic mitochondrial dysfunction. J Biol Chem. 1979 Oct 10;254(19):9871–9878. [PubMed] [Google Scholar]
- Moore L., Rodman Davenport G., Landon E. J. Calcium uptake of a rat liver microsomal subcellular fraction in response to in vivo administration of carbon tetrachloride. J Biol Chem. 1976 Feb 25;251(4):1197–1201. [PubMed] [Google Scholar]
- Murphy E., Coll K., Rich T. L., Williamson J. R. Hormonal effects on calcium homeostasis in isolated hepatocytes. J Biol Chem. 1980 Jul 25;255(14):6600–6608. [PubMed] [Google Scholar]
- PLACER Z., VESELKOVA A., RATH R. KINETIK DES MALONDIALDEHYDES IM ORGANISMUS. Experientia. 1965 Jan 15;21:19–20. doi: 10.1007/BF02136359. [DOI] [PubMed] [Google Scholar]
- Penttila A., Trump B. F. Studies on modification on the cellular response to injury. Lab Invest. 1975 Jun;32(6):690–695. [PubMed] [Google Scholar]
- Penttila A., Trump B. F. Studies on the modification of the cellular response to injury. III. Electron microscopic studies on the protective effect of acidosis on p-chloromercuribenzene sulfonic acid-(PCMBS) induced injury of Ehrlich ascites tumor cells. Virchows Arch B Cell Pathol. 1975;18(1):17–34. [PubMed] [Google Scholar]
- Poli G., Gravela E., Albano E., Dianzani M. U. Studies on fatty liver with isolated hepatocytes. II. The action of carbon tetrachloride on lipid peroxidation, protein, and triglyceride synthesis and secretion. Exp Mol Pathol. 1979 Feb;30(1):116–127. doi: 10.1016/0014-4800(79)90086-8. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. LIVER PARENCHYMAL CELL INJURY. I. INITIAL ALTERATIONS OF THE CELL FOLLOWING POISONING WITH CARBON TETRACHLORIDE. J Cell Biol. 1963 Oct;19:139–157. doi: 10.1083/jcb.19.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. LIVER PARENCHYMAL CELL INJURY. II. CYTOCHEMICAL EVENTS CONCERNED WITH MITOCHONDRIAL DYSFUNCTION FOLLOWING POISONING WITH CARBON TETRACHLORIDE. Lab Invest. 1964 Nov;13:1457–1470. [PubMed] [Google Scholar]
- Rechnagel R. O., Glende E. A., Jr Carbon tetrachloride hepatotoxicity: an example of lethal cleavage. CRC Crit Rev Toxicol. 1973 Nov;2(3):263–297. doi: 10.3109/10408447309082019. [DOI] [PubMed] [Google Scholar]
- Recknagel R. O., Ghoshal A. K. New data on the question of lipoperoxidation in carbon tetrachloride poisoning. Exp Mol Pathol. 1966 Apr;5(2):108–117. doi: 10.1016/0014-4800(66)90008-6. [DOI] [PubMed] [Google Scholar]
- Roders M. K., Glende E. A., Jr, Recknagel R. O. Prelytic damage of red cells in filtrates from peroxidizing microsomes. Science. 1977 Jun 10;196(4295):1221–1222. doi: 10.1126/science.16344. [DOI] [PubMed] [Google Scholar]
- Roders M. K., Glende E. A., Recknagel R. O. NADPH-dependent microsomal lipid peroxidation and the problem of pathological action at a distance. New data on induction of red cell damage. Biochem Pharmacol. 1978 Feb 15;27(4):437–443. doi: 10.1016/0006-2952(78)90373-8. [DOI] [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Schanne F. A., Pfau R. G., Farber J. L. Galactosamine-induced cell death in primary cultures of rat hepatocytes. Am J Pathol. 1980 Jul;100(1):25–38. [PMC free article] [PubMed] [Google Scholar]
- Smuckler E. A. Studies on carbon tetrachloride intoxication. IV. Effect of carbon tetrachloride on liver slices and isolated organelles in vitro. Lab Invest. 1966 Jan;15(1 Pt 1):157–166. [PubMed] [Google Scholar]
- Stacey N. H., Cantilena L. R., Jr, Klaassen C. D. Cadmium toxicity and lipid peroxidation in isolated rat hepatocytes. Toxicol Appl Pharmacol. 1980 May;53(3):470–480. doi: 10.1016/0041-008x(80)90359-2. [DOI] [PubMed] [Google Scholar]
- Stacey N., Priestly B. G. Lipid peroxidation in isolated rat hepatocytes: relationship to toxicity of CCl4, ADP/Fe3+, and diethyl maleate. Toxicol Appl Pharmacol. 1978 Jul;45(1):41–48. doi: 10.1016/0041-008x(78)90026-1. [DOI] [PubMed] [Google Scholar]
- Taylor W. M., Bygrave F. L., Blackmore P. F., Exton J. H. Stable enhancement of ruthenium red-insensitive calcium transport in an endoplasmic reticulum-rich fraction following the exposure of isolated rat liver cells to glucagon. FEBS Lett. 1979 Aug 1;104(1):31–34. doi: 10.1016/0014-5793(79)81079-0. [DOI] [PubMed] [Google Scholar]
- Trump B. F., Laiho K. A., Mergner W. J., Arstila A. U. Studies on the subcellular pathophysiology of acute lethal cell injury. Beitr Pathol. 1974;152(3):243–271. doi: 10.1016/s0005-8165(74)80177-0. [DOI] [PubMed] [Google Scholar]
- Wands J. R., Smuckler E. A., Woodbury W. J. Transmembrane potential changes in liver cells following CCl4 intoxication. Am J Pathol. 1970 Mar;58(3):499–508. [PMC free article] [PubMed] [Google Scholar]
- Weddle C. C., Hornbrook K. R., McCay P. B. Lipid peroxidation and alteration of membrane lipids in isolated hepatocytes exposed to carbon tetrachloride. J Biol Chem. 1976 Aug 25;251(16):4973–4978. [PubMed] [Google Scholar]


