Abstract
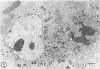
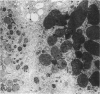
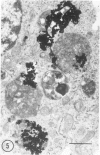
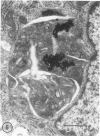
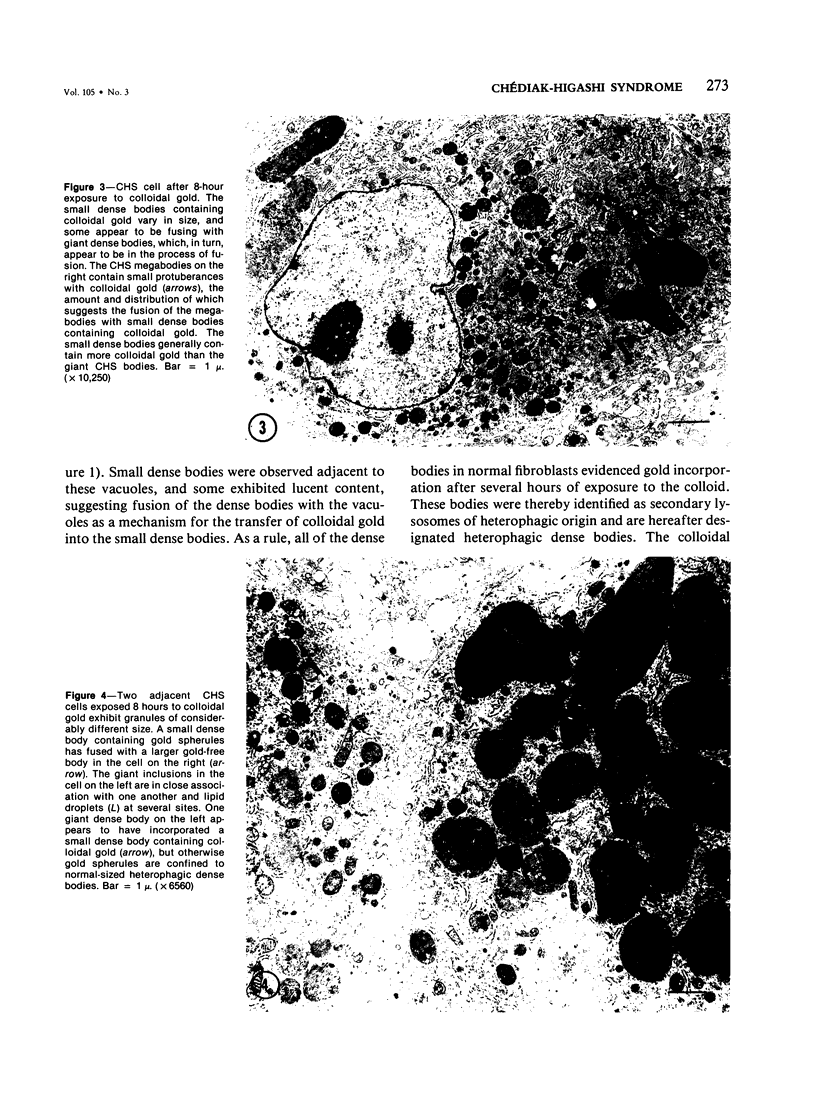
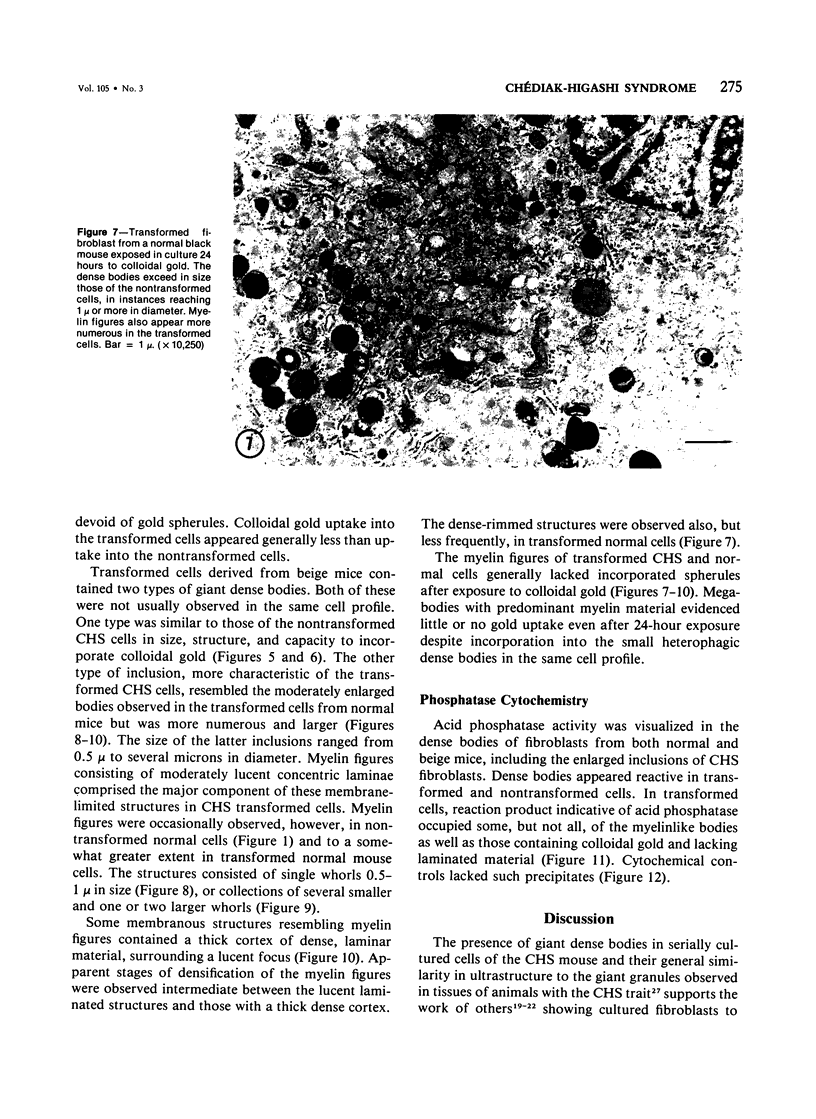
Fibroblasts cultured from the skin of beige mice manifesting the Chédiak-Higashi syndrome (CHS), unlike cells derived from normal black mice, exhibited giant dense bodies in the cytoplasm. These megabodies were membrane-delimited and exhibited dense content by electron microscopy, with myelin figures, highly osmiophilic, thick membranous contours, and lucent areas. The megabodies evidenced acid phosphatase ultrastructurally. Cells of both normal and CHS mice contained smaller dense bodies. During a 2--6 hour exposure to colloidal gold, the smaller dense bodies of normal and CHS fibroblasts selectively incorporated the gold spherules and, accordingly, were identified as secondary lysosomes of heterophagic origin. With longer exposure to colloidal gold, the small dense bodies of the normal and CHS cells disclosed increased content of colloidal gold. After 24 hour exposure to colloidal gold, many giant dense bodies also exhibited gold particles, evidencing uptake of endocytosed material into the giant structures and the heterophagic origin of at least some of the content of the bodies. The gold spherules initially incorporated into the giant dense bodies were concentrated in foci along their periphery and indicated fusion of small dense bodies into the giant structures. Transformed normal and CHS cells appeared to contain more abundant myelin figures than nontransformed cells, and these were larger in transformed CHS cells and constituted a major component of their giant dense bodies. The giant inclusions of the transformed CHS cells generally contained little colloidal gold, suggesting their derivation principally through cellular autophagy.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. M., Blume R. S., Wolff S. M. Characterization and significance of abnormal leukocyte granules in the beige mouse: a possible homologue for Chediak-Higashi Aleutian trait. J Lab Clin Med. 1969 Feb;73(2):235–243. [PubMed] [Google Scholar]
- Blume R. S., Wolff S. M. The Chediak-Higashi syndrome: studies in four patients and a review of the literature. Medicine (Baltimore) 1972 Jul;51(4):247–280. [PubMed] [Google Scholar]
- CHEDIAK M. M. Nouvelle anomalie leucocytaire de caractère constitutionnel et familial. Rev Hematol. 1952;7(3):362–367. [PubMed] [Google Scholar]
- Chi E. Y., Lagunoff D. Abnormal mast cell granules in the beige (Chédiak-Higashi syndrome) mouse. J Histochem Cytochem. 1975 Feb;23(2):117–122. doi: 10.1177/23.2.46876. [DOI] [PubMed] [Google Scholar]
- Chi E. Y., Lagunoff D., Koehler J. K. Abnormally large lamellar bodies in type II pneumocytes in Chediak-Higashi syndrome in beige mice. Lab Invest. 1976 Feb;34(2):166–173. [PubMed] [Google Scholar]
- Danes B. S., Bearn A. G. Cell culture and the Chediak-Higashi syndrome. Lancet. 1967 Jul 8;2(7506):65–67. doi: 10.1016/s0140-6736(67)92059-4. [DOI] [PubMed] [Google Scholar]
- Davis W. C., Spicer S. S., Greene W. B., Padgett G. A. Ultrastructure of bone marrow granulocytes in normal mink and mink with the homolog of the Chediak-Higashi trait of humans. I. Origin of the abnormal granules present in the neutrophils of mink with the C-HS trait. Lab Invest. 1971 Apr;24(4):303–317. [PubMed] [Google Scholar]
- Davis W. C., Spicer S. S., Greene W. B., Padgett G. A. Ultrastructure of cells in bone marrow and peripheral blood of normal mink and mink with the homologue of the Chediak-Higashi trait of humans. II. Cytoplasmic granules in eosinophils, basophils, mononuclear cells and platelets. Am J Pathol. 1971 Jun;63(3):411–432. [PMC free article] [PubMed] [Google Scholar]
- Dent P. B., Fish L. A., White L. G., Good R. A. Chediak-Higashi syndrome. Observations on the nature of the associated malignancy. Lab Invest. 1966 Oct;15(10):1634–1642. [PubMed] [Google Scholar]
- Essner E., Oliver C. A hereditary alteration in kidneys of mice with Chediak-Higashi syndrome. Am J Pathol. 1973 Oct;73(1):217–232. [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R., Tucker R. W., Bruce J., Stenberg R. Fibroblasts and macrophages of mice with the Chediak-Higashi-like syndrome have microtubules and actin cables. J Cell Biol. 1978 Nov;79(2 Pt 1):401–408. doi: 10.1083/jcb.79.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms E., Kern H., Schneider J. A. Human lysosomes can be purified from diploid skin fibroblasts by free-flow electrophoresis. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6139–6143. doi: 10.1073/pnas.77.10.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRITZLER R. A., TERNER J. Y., LINDENBAUM J., MAGIDSON J., WILLIAMS R., PRESIG R., PHILLIPS G. B. CHEDIAK-HIGASHI SYNDROME. CYTOLOGIC AND SERUM LIPID OBSERVATIONS IN A CASE AND FAMILY. Am J Med. 1964 Apr;36:583–594. doi: 10.1016/0002-9343(64)90106-8. [DOI] [PubMed] [Google Scholar]
- Kontermann K., Bayreuther K. The cellular aging of rat fibroblasts in vitro is a differentiation process. Gerontology. 1979;25(5):261–274. doi: 10.1159/000212351. [DOI] [PubMed] [Google Scholar]
- Kramer J. W., Davis W. C., Prieur D. J. The Chediak-Higashi syndrome of cats. Lab Invest. 1977 May;36(5):554–562. [PubMed] [Google Scholar]
- Oliver J. M., Krawiec J. A., Berlin R. D. Carbamycholine prevents giant granule-formation in cultured fibroblasts from beige (Chediak-Higashi) mice. J Cell Biol. 1976 Apr;69(1):205–210. doi: 10.1083/jcb.69.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PADGETT G. A., LEADER R. W., GORHAM J. R., O'MARY C. C. THE FAMILIAL OCCURRENCE OF THE CHEDIAK-HIGASHI SYNDROME IN MINK AND CATTLE. Genetics. 1964 Mar;49:505–512. doi: 10.1093/genetics/49.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieur D. J., Davis W. C., Padgett G. A. Defective function of renal lysosomes in mice with the Chediak-Higashi syndrome. Am J Pathol. 1972 May;67(2):227–236. [PMC free article] [PubMed] [Google Scholar]
- Rome L. H., Garvin A. J., Allietta M. M., Neufeld E. F. Two species of lysosomal organelles in cultured human fibroblasts. Cell. 1979 May;17(1):143–153. doi: 10.1016/0092-8674(79)90302-7. [DOI] [PubMed] [Google Scholar]
- SWIFT H., HRUBAN Z. FOCAL DEGRADATION AS A BIOLOGICAL PROCESS. Fed Proc. 1964 Sep-Oct;23:1026–1037. [PubMed] [Google Scholar]
- Spicer S. S., Prioleau W. H., Jr Cytochemical studies of lipid and acid phosphatase in the human eccrine sweat gland. Lab Invest. 1972 Jul;27(1):9–16. [PubMed] [Google Scholar]
- Spicer S. S., Sannes P. L., Eguchi M., McKeever P. E. Fine structural and cytochemical aspects of the development of macrophages and of their endocytic and secretory activity. J Reticuloendothel Soc. 1979 Jul;26(1):49–65. [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter N. L. Electron-opaque, lipid-containing bodies in mouse liver at early intervals after partial hepatectomy and sham operation. J Cell Biol. 1965 Jun;25(3 Suppl):41–52. doi: 10.1083/jcb.25.3.41. [DOI] [PubMed] [Google Scholar]
- White J. G., Clawson C. C. The Chédiak-Higashi syndrome; the nature of the giant neutrophil granules and their interactions with cytoplasm and foreign particulates. I. Progressive enlargement of the massive inclusions in mature neutrophils. II. Manifestations of cytoplasmic injury and sequestration. III. Interactions between giant organelles and foreign particulates. Am J Pathol. 1980 Jan;98(1):151–196. [PMC free article] [PubMed] [Google Scholar]
- White J. G. The Chediak-Higashi syndrome: a possible lysosomal disease. Blood. 1966 Aug;28(2):143–156. [PubMed] [Google Scholar]