Abstract
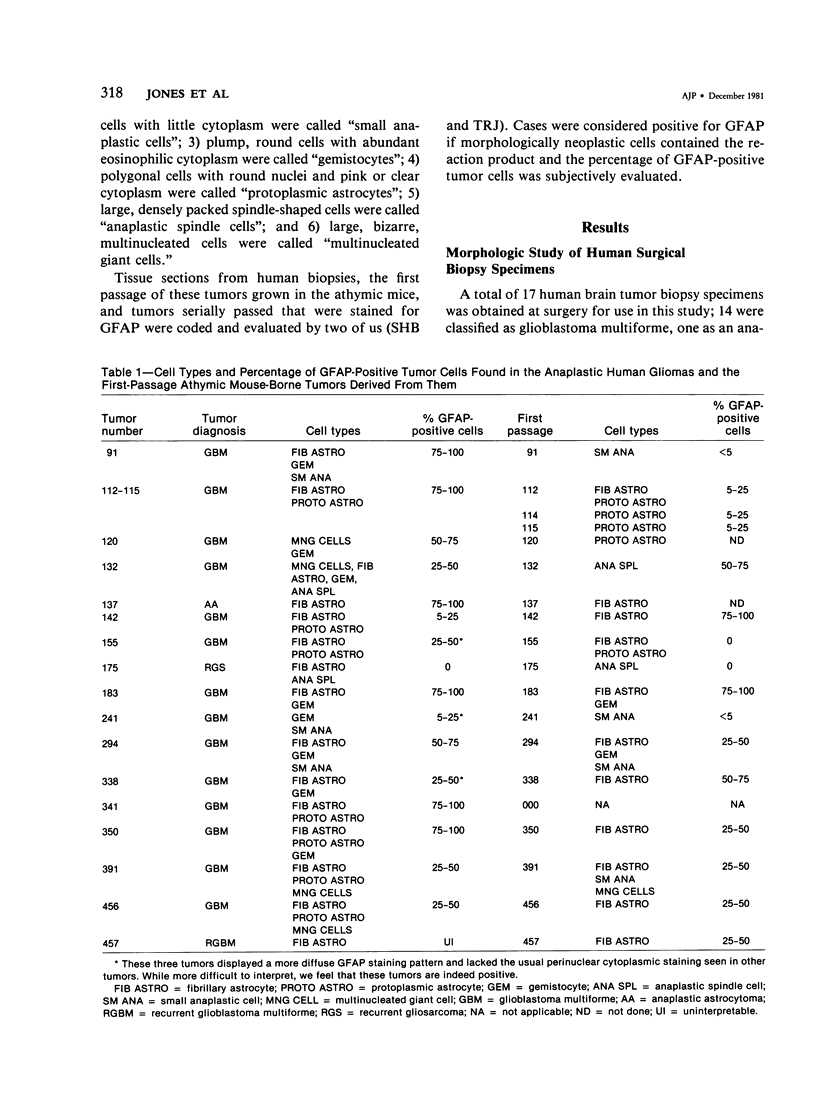
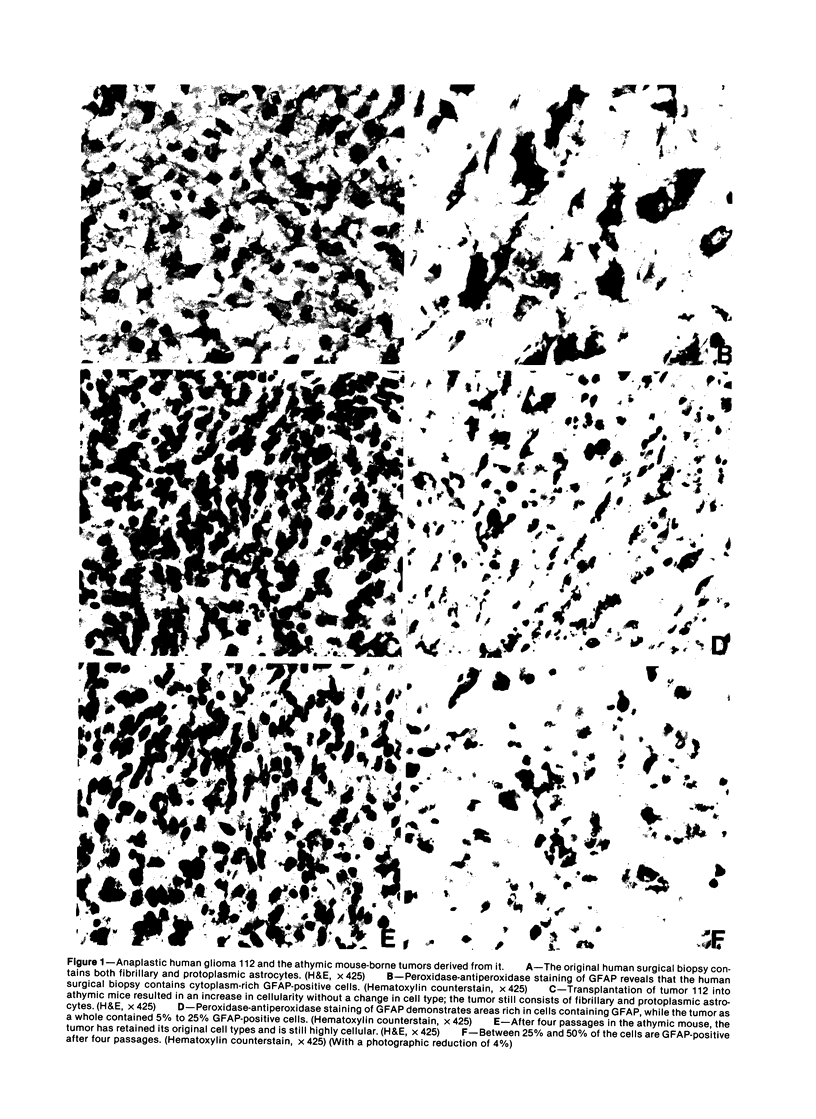
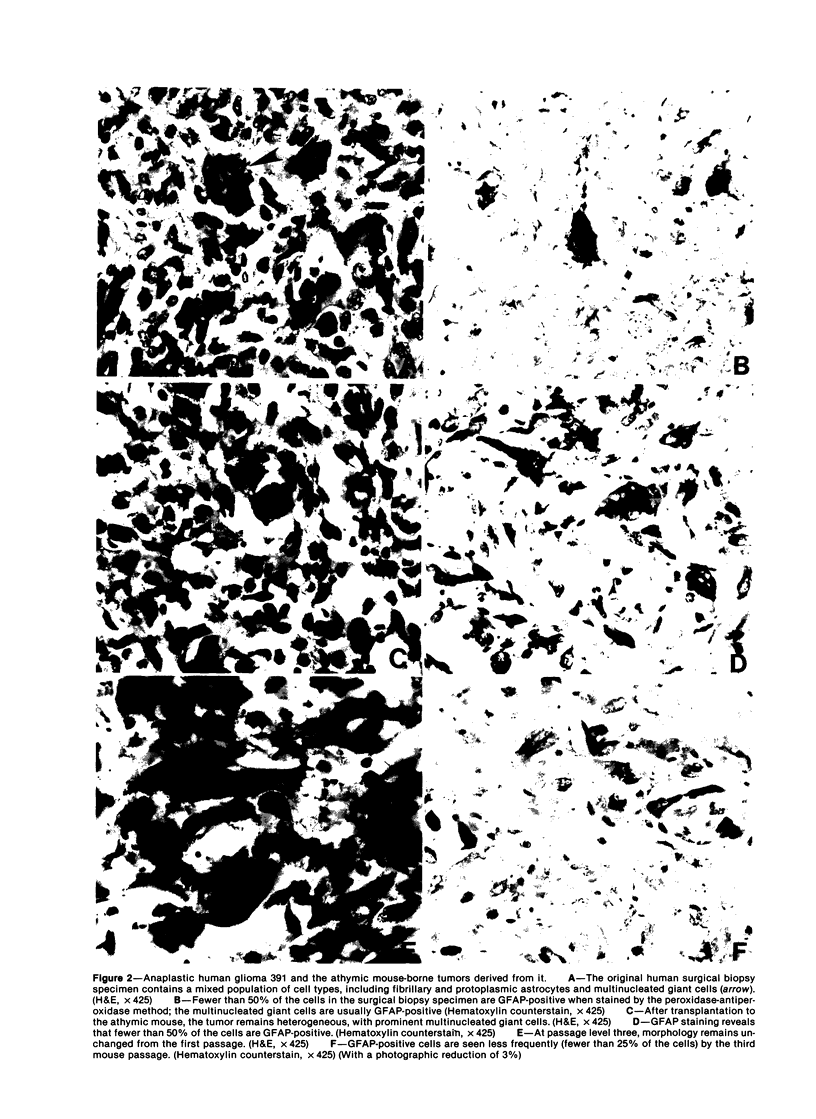
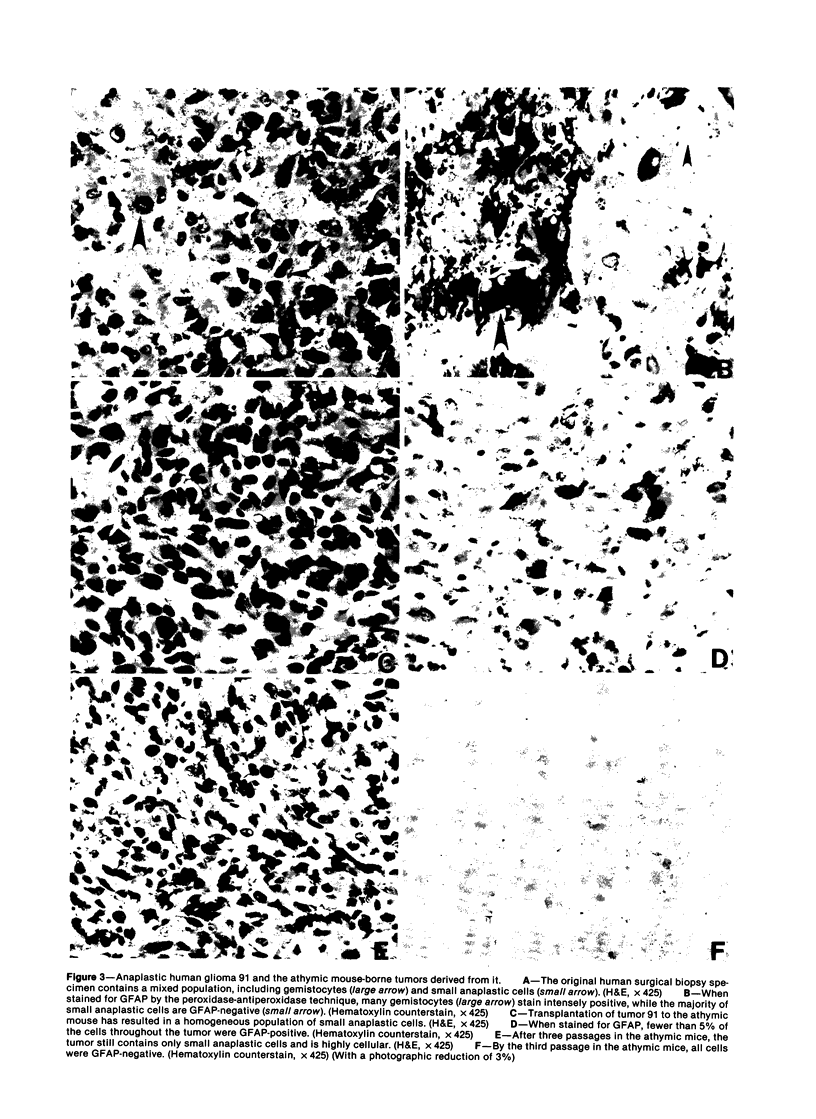
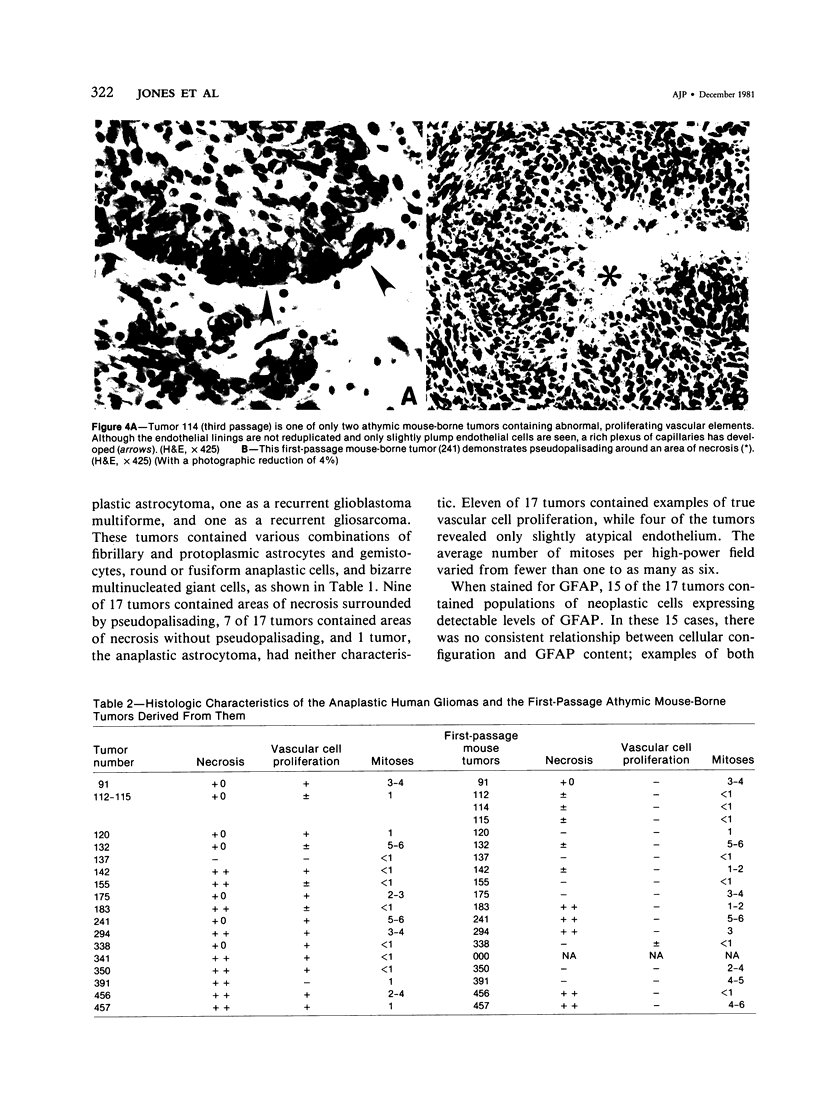
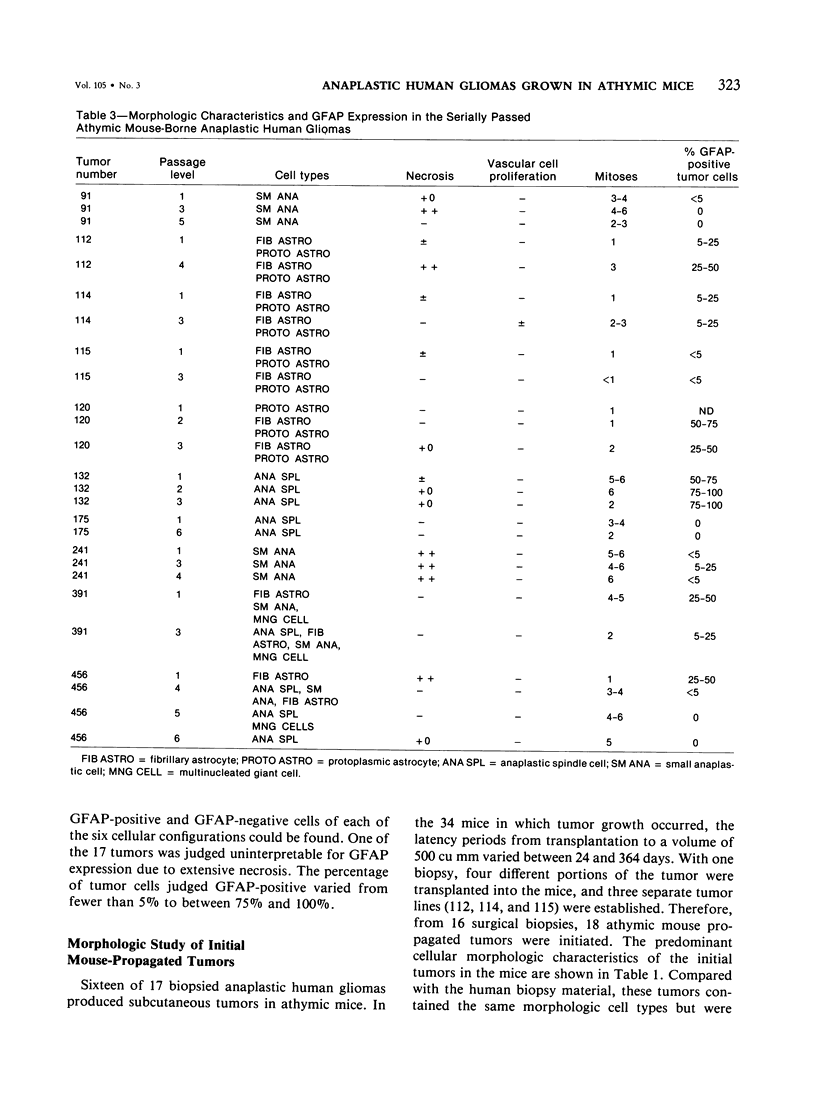
The morphologic and biochemical characteristics of human surgical biopsy specimens taken from 17 patients with anaplastic human gliomas and of athymic mouse-grown tumors derived from them were examined. Fourteen were categorized as glioblastoma multiforme, one as an anaplastic astrocytoma, one as a recurrent glioblastoma multiforme, and one as a gliosarcoma. Fifteen of 17 tumors stained positively immunohistochemically for glial fibrillary acidic protein (GFAP), a glial-specific marker. When portions of the 17 surgical biopsy specimens were injected into the flank subcutaneous space of athymic mice, 16 produced tumors; different portions of a single biopsy specimen were used to establish three separate tumor lines; in toto, 18 tumor lines were established. Mouse-borne tumors contained various proportions of fibrillary and protoplasmic astrocytes, gemistocytes, small anaplastic cells, and multinucleated giant cells. Some were more homogeneous than the human tumors from which they were derived, while others contained a mixed population similar to that of the original biopsy specimen. Of these initial 18 tumors, 16 were stained for GFAP and 14 contained from fewer than 5% to almost 100% GFAP-expressing cells. Ten of the tumor lines were studied in serial passage, several demonstrating increased cellularity with increased passage. GFAP expression was followed through serial passage, and 7 of 10 tumor lines continued to express it, often in reduced amounts, and 2 of 10 ceased expression, one (the gliosarcoma) never having expressed it. These data demonstrate that while athymic mouse-borne human anaplastic gliomas retained some features of the human tumors from which they were derived, they varied from one another morphologically. These mouse-borne tumors also continued to evolve, often changing their levels of GFAP and demonstrating increased cellularity with passage.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedict W. F., Dawson J. A., Banerjee A., Murphree A. L. The nude mouse model for human retinoblastoma: a system for evaluation of retinoblastoma therapy. Med Pediatr Oncol. 1980;8(4):391–395. doi: 10.1002/mpo.2950080411. [DOI] [PubMed] [Google Scholar]
- Bigner D. D., Bigner S. H., Pontén J., Westermark B., Mahaley M. S., Ruoslahti E., Herschman H., Eng L. F., Wikstrand C. J. Heterogeneity of Genotypic and phenotypic characteristics of fifteen permanent cell lines derived from human gliomas. J Neuropathol Exp Neurol. 1981 May;40(3):201–229. doi: 10.1097/00005072-198105000-00001. [DOI] [PubMed] [Google Scholar]
- Bullard D. E., Schold S. C., Jr, Bigner S. H., Bigner D. D. Growth and chemotherapeutic response in athymic mice of tumors arising from human glioma-derived cell lines. J Neuropathol Exp Neurol. 1981 Jul;40(4):410–427. doi: 10.1097/00005072-198107000-00005. [DOI] [PubMed] [Google Scholar]
- Deck J. H., Eng L. F., Bigbee J., Woodcock S. M. The role of glial fibrillary acidic protein in the diagnosis of central nervous system tumors. Acta Neuropathol. 1978 Jun 30;42(3):183–190. doi: 10.1007/BF00690355. [DOI] [PubMed] [Google Scholar]
- Dittmann L., Axelsen N. H., Norgaard-Pedersen B., Bock E. Antigens in human glioblastomas and meningiomas: Search for tumour and onco-foetal antigens. Estimation of S-100 and GFA protein. Br J Cancer. 1977 Feb;35(2):135–141. doi: 10.1038/bjc.1977.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy P. E., Graf L., Rapport M. M. Identification of glial fibrillary acidic protein by the immunoperoxidase method in human brain tumors. J Neuropathol Exp Neurol. 1977 Jul;36(4):645–652. doi: 10.1097/00005072-197707000-00001. [DOI] [PubMed] [Google Scholar]
- Eng L. F., Rubinstein L. J. Contribution of immunohistochemistry to diagnostic problems of human cerebral tumors. J Histochem Cytochem. 1978 Jul;26(7):513–522. doi: 10.1177/26.7.357640. [DOI] [PubMed] [Google Scholar]
- Giovanella B. C., Stehlin J. S., Jr, Williams L. J., Jr, Lee S. S., Shepard R. C. Heterotransplantation of human cancers into nude mice: a model system for human cancer chemotherapy. Cancer. 1978 Nov;42(5):2269–2281. doi: 10.1002/1097-0142(197811)42:5<2269::aid-cncr2820420527>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Jacque C. M., Vinner C., Kujas M., Raoul M., Racadot J., Baumann N. A. Determination of glial fibrillary acidic protein (GFAP) in human brain tumors. J Neurol Sci. 1978 Jan;35(1):147–155. doi: 10.1016/0022-510x(78)90107-7. [DOI] [PubMed] [Google Scholar]
- Maunoury R., Courdi A., Vedrenne C., Constans J. P. Localisation immunocytochimique de la protéine gliofibrillaire dans les hétérogreffes de cultures de gliomes humains. Neurochirurgie. 1978;24(4):221–226. [PubMed] [Google Scholar]
- Ovejera A. A., Houchens D. P., Barker A. D. Chemotherapy of human tumor xenografts in genetically athymic mice. Ann Clin Lab Sci. 1978 Jan-Feb;8(1):50–56. [PubMed] [Google Scholar]
- Palfreyman J. W., Thomas D. G., Ratcliffe J. G., Graham D. I. Glial fibrillary acidic protein (GFAP): purification from human fibrillary astrocytoma, development and validation of a radioimmunoassay for GFAP-like immunoactivity. J Neurol Sci. 1979 Mar;41(1):101–113. doi: 10.1016/0022-510x(79)90144-8. [DOI] [PubMed] [Google Scholar]
- Pantelouris E. M. Absence of thymus in a mouse mutant. Nature. 1968 Jan 27;217(5126):370–371. doi: 10.1038/217370a0. [DOI] [PubMed] [Google Scholar]
- Povlsen C. O., Visfeldt J., Rygaard J., Jensen G. Growth patterns and chromosome constitutions of human malignant tumours after long-term serial transplantation in nude mice. Acta Pathol Microbiol Scand A. 1975 Nov;83(6):709–716. doi: 10.1111/j.1699-0463.1975.tb01401.x. [DOI] [PubMed] [Google Scholar]
- Rana M. W., Pinkerton H., Thornton H., Nagy D. Heterotransplantation of human glioblastoma multiforme and meningioma to nude mice. Proc Soc Exp Biol Med. 1977 May;155(1):85–88. doi: 10.3181/00379727-155-39750. [DOI] [PubMed] [Google Scholar]
- Rasmussen S., Bock E., Warecka K., Althage G. Quantitation of glial fibrillary acidic protein in human brain tumours. Br J Cancer. 1980 Jan;41(1):113–116. doi: 10.1038/bjc.1980.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygaard J., Povlsen C. O. Heterotransplantation of a human malignant tumour to "Nude" mice. Acta Pathol Microbiol Scand. 1969;77(4):758–760. doi: 10.1111/j.1699-0463.1969.tb04520.x. [DOI] [PubMed] [Google Scholar]
- Shapiro J. R., Yung W. K., Shapiro W. R. Isolation, karyotype, and clonal growth of heterogeneous subpopulations of human malignant gliomas. Cancer Res. 1981 Jun;41(6):2349–2359. [PubMed] [Google Scholar]
- Shapiro W. R., Basler G. A., Chernik N. L., Posner J. B. Human brain tumor transplantation into nude mice. J Natl Cancer Inst. 1979 Mar;62(3):447–453. doi: 10.1093/jnci/62.3.447. [DOI] [PubMed] [Google Scholar]
- Velasco M. E., Dahl D., Roessmann U., Gambetti P. Immunohistochemical localization of glial fibrillary acidic protein in human glial neoplasms. Cancer. 1980 Feb;45(3):484–494. doi: 10.1002/1097-0142(19800201)45:3<484::aid-cncr2820450312>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Yamashita M., Egami H., Mannoji H., Ohta M., Kitamura K. Alternate culture and animal passage of human glioma. Acta Neuropathol. 1981;53(1):35–40. doi: 10.1007/BF00697182. [DOI] [PubMed] [Google Scholar]
- de Armond S. J., Eng L. F., Rubinstein L. J. The application of glial fibrillary acidic (GFA) protein immunohistochemistry in neurooncology. A progress report. Pathol Res Pract. 1980;168(4):374–394. doi: 10.1016/s0344-0338(80)80273-1. [DOI] [PubMed] [Google Scholar]
- van der Meulen J. D., Houthoff H. J., Ebels E. J. Glial fibrillary acidic protein in human gliomas. Neuropathol Appl Neurobiol. 1978 May-Jun;4(3):177–190. doi: 10.1111/j.1365-2990.1978.tb00534.x. [DOI] [PubMed] [Google Scholar]






