Abstract
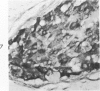
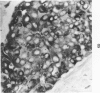
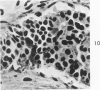
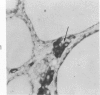
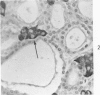
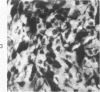
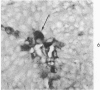
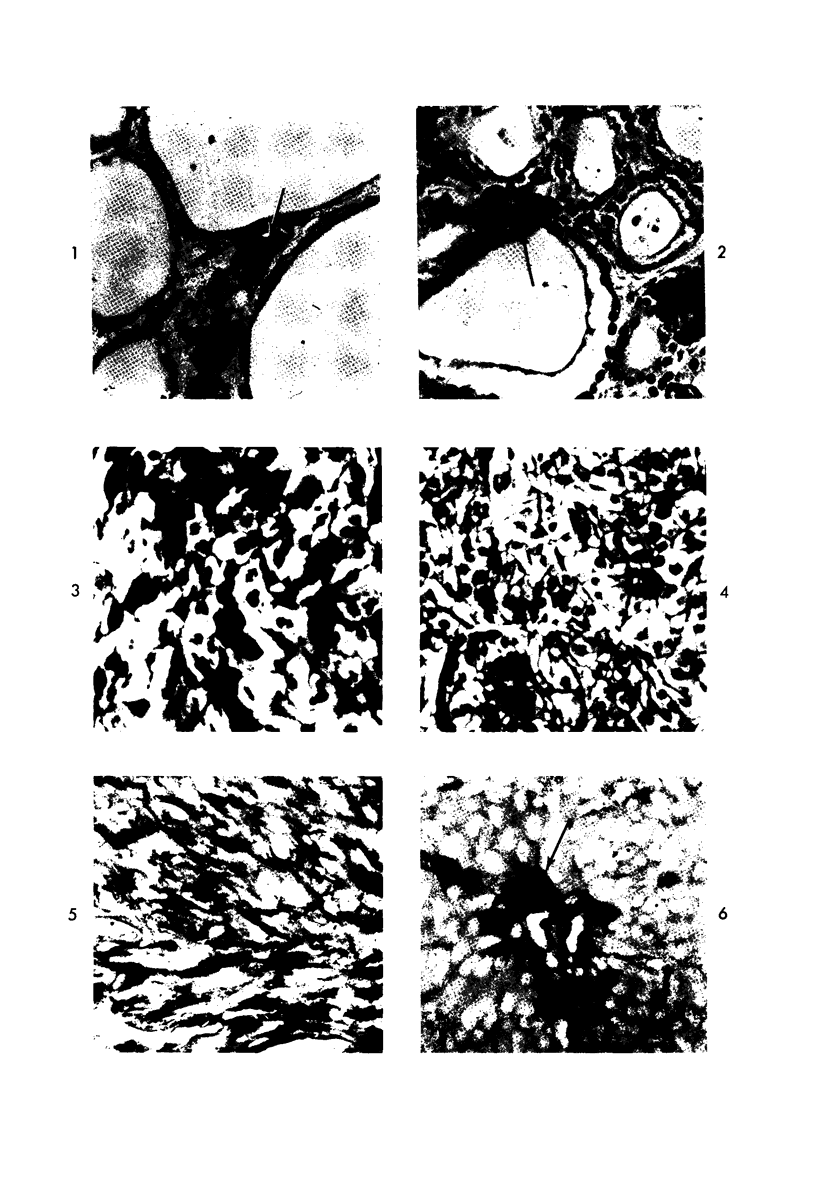
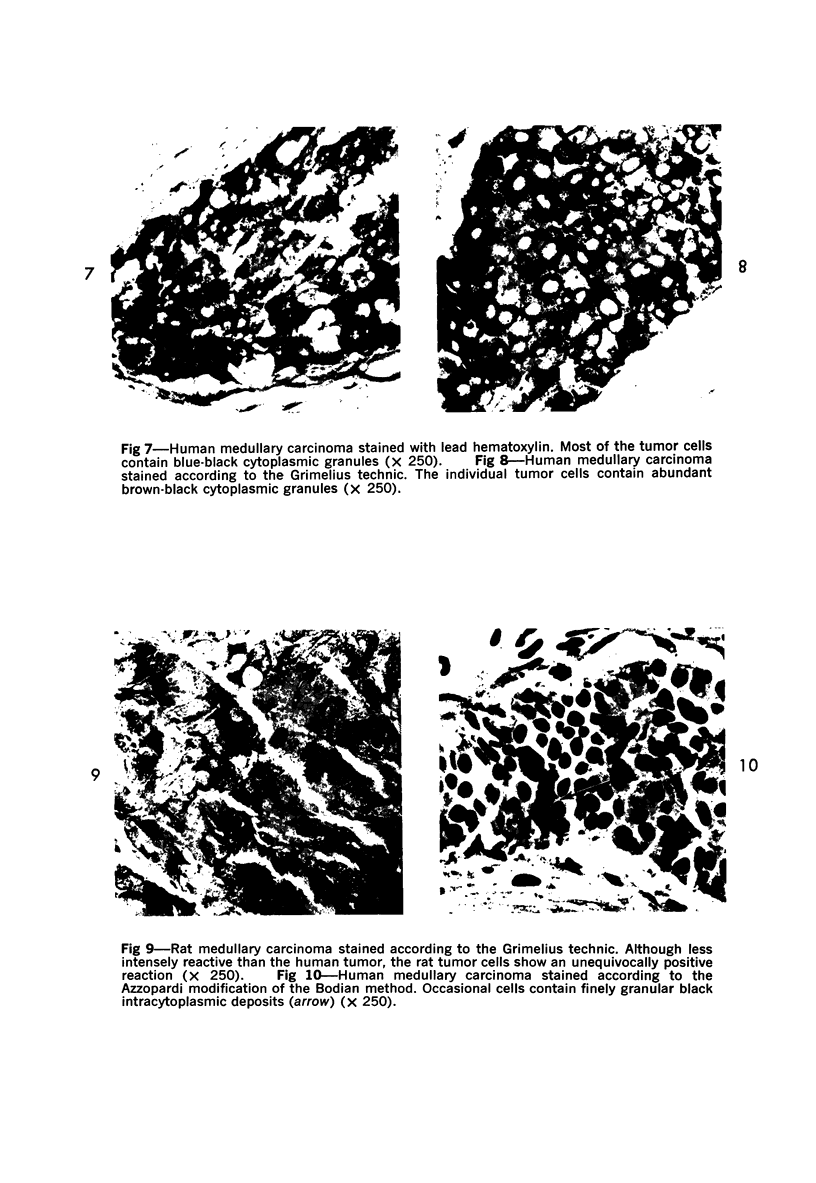
Normal canine parafollicular cells share a common set of histochemical characteristics, which include masked metachromasia and argyrophilia, with human as well as rat medullary thyroid carcinomas. Masked metachromasia, a property of polypeptide hormone-producing cells, was demonstrated by staining with toluidine blue or coriophosphine O after acid hydrolysis. Argyrophilia was demonstrated both by the Grimelius silver nitrate technic and by the Azzopardi modification of the Bodian method. With these technics, parafollicular cells in the dog and medullary carcinoma of human and rat origin showed nearly identical staining reactions. Fixation in glutaraldehyde was superior to formaldehyde in demonstrating masked metachromasia of normal dog parafollicular cells, while formaldehyde fixation was superior for the demonstration of argyrophilia of granules. In general, the Grimelius method was superior to the Azzopardi modification of the Bodian technic for the demonstration of argyophilia. The results of this study provided further support for the parafollicular cell origin of human and rat medullary thyroid carcinomas and also provided a useful set of histochemical criteria for the diagnosis of this neoplasm.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AZZOPARDI J. G., POLLOCK D. J. ARGENTAFFIN AND ARGYROPHIL CELLS IN GASTRIC CARCINOMA. J Pathol Bacteriol. 1963 Oct;86:443–451. doi: 10.1002/path.1700860219. [DOI] [PubMed] [Google Scholar]
- Boorman G. A., van Noord M. J., Hollander C. F. Naturally occurring medullary thyroid carcinoma in the rat. Arch Pathol. 1972 Jul;94(1):35–41. [PubMed] [Google Scholar]
- Bussolati G., Rost F. W., Pearse A. G. Fluorescence metachromasia in polypeptide hormone-producing cells of the APUD series, and its significance in relation to the structure of the precursor protein. Histochem J. 1969 Nov;1(6):517–530. doi: 10.1007/BF01012858. [DOI] [PubMed] [Google Scholar]
- Grimelius L. A silver nitrate stain for alpha-2 cells in human pancreatic islets. Acta Soc Med Ups. 1968;73(5-6):243–270. [PubMed] [Google Scholar]
- Grimley P. M., Deftos L. J., Weeks J. R., Rabson A. S. Growth in vitro and ultrastructure of cells from a medullary carcinoma of the human thyroid gland: transformation by simian virus 40 and evidenc of thyrocalcitonin and prostaglandins. J Natl Cancer Inst. 1969 Apr;42(4):663–680. [PubMed] [Google Scholar]
- HAZARD J. B., HAWK W. A., CRILE G., Jr Medullary (solid) carcinoma of the thyroid; a clinicopathologic entity. J Clin Endocrinol Metab. 1959 Jan;19(1):152–161. doi: 10.1210/jcem-19-1-152. [DOI] [PubMed] [Google Scholar]
- Lindsay S., Nichols C. W. Medullary thyroid carcinoma and parathyroid hyperplasia in rats. Arch Pathol. 1969 Oct;88(4):402–406. [PubMed] [Google Scholar]
- Ljungberg O. Argentaffin cells in human thyroid and parathyroid and their relationship to c-cells and medullary carcinoma. Acta Pathol Microbiol Scand A. 1972;80(5):589–599. doi: 10.1111/j.1699-0463.1972.tb00320.x. [DOI] [PubMed] [Google Scholar]
- Ljungberg O. Cresyl fast violet--a selective stain for human C-cells. Acta Pathol Microbiol Scand A. 1970;78(5):618–620. doi: 10.1111/j.1699-0463.1970.tb02547.x. [DOI] [PubMed] [Google Scholar]
- Ljungberg O., Dymling J. F. Pathogenesis of C-cell neoplasia in thyroid gland. C-cell proliferation in a case of chronic hypercalcaemia. Acta Pathol Microbiol Scand A. 1972;80(5):577–588. doi: 10.1111/j.1699-0463.1972.tb00319.x. [DOI] [PubMed] [Google Scholar]
- Ljungberg O. Two cell types in familial medullary thyroid carcinoma. A histochemical study. Virchows Arch A Pathol Pathol Anat. 1970;349(4):312–322. doi: 10.1007/BF00543183. [DOI] [PubMed] [Google Scholar]
- Meyer J. S., Abdel-Bari W. Granules and thyrocalcitonin-like activity in medullary carcinoma of the thyroid gland. N Engl J Med. 1968 Mar 7;278(10):523–529. doi: 10.1056/NEJM196803072781002. [DOI] [PubMed] [Google Scholar]
- Pearse A. G. Common cytochemical and ultrastructural characteristics of cells producing polypeptide hormones (the APUD series) and their relevance to thyroid and ultimobranchial C cells and calcitonin. Proc R Soc Lond B Biol Sci. 1968 May 14;170(1018):71–80. doi: 10.1098/rspb.1968.0025. [DOI] [PubMed] [Google Scholar]
- Pearse A. G. Random coil conformation of polypeptide hormone precursor protein in endocrine cells. Nature. 1969 Mar 29;221(5187):1210–1211. doi: 10.1038/2211210a0. [DOI] [PubMed] [Google Scholar]
- Solcia E., Capella C., Vassallo G. Lead-haematoxylin as a stain for endocrine cells. Significance of staining and comparison with other selective methods. Histochemie. 1969;20(2):116–126. doi: 10.1007/BF00268705. [DOI] [PubMed] [Google Scholar]
- Solcia E., Vassallo G., Capella C. Selective staining of endocrine cells by basic dyes after acid hydrolysis. Stain Technol. 1968 Sep;43(5):257–263. doi: 10.3109/10520296809115078. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Melvin E. W. Medullary carcinoma of the thyroid gland. Studies of thyrocalcitonin in plasma and tumor extracts. N Engl J Med. 1968 Aug 8;279(6):279–283. doi: 10.1056/NEJM196808082790602. [DOI] [PubMed] [Google Scholar]
- Tateishi R., Takahashi Y., Noguchi A. Histologic and ultracytochemical studies on thyroid medullary carcinoma. Diagnostic significance of argyrophil secretory granules. Cancer. 1972 Sep;30(3):755–763. doi: 10.1002/1097-0142(197209)30:3<755::aid-cncr2820300325>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Teitlebaum S. L., Moore K. E., Shieber W. Parafollicular cells in the normal human thyroid. Nature. 1971 Apr 2;230(5292):334–335. doi: 10.1038/230334a0. [DOI] [PubMed] [Google Scholar]