Abstract
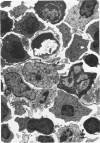
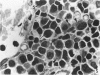
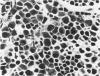
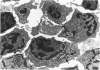
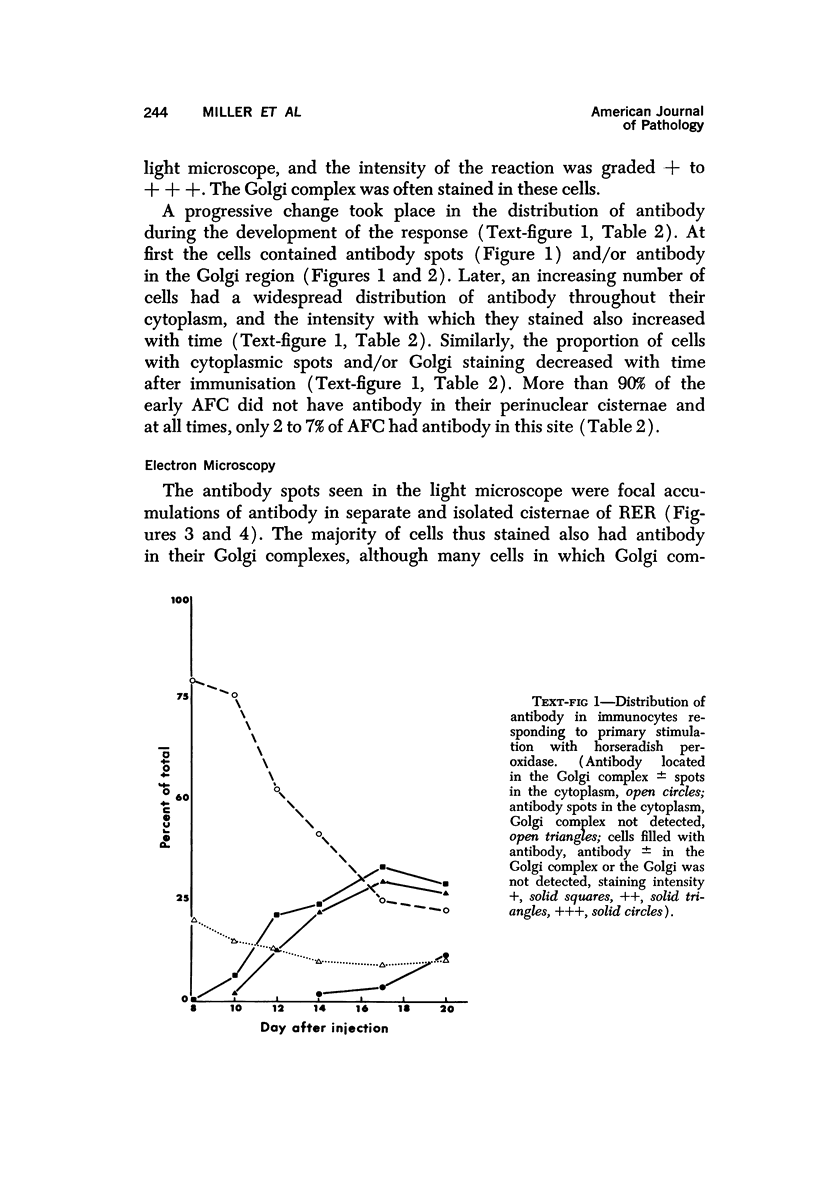
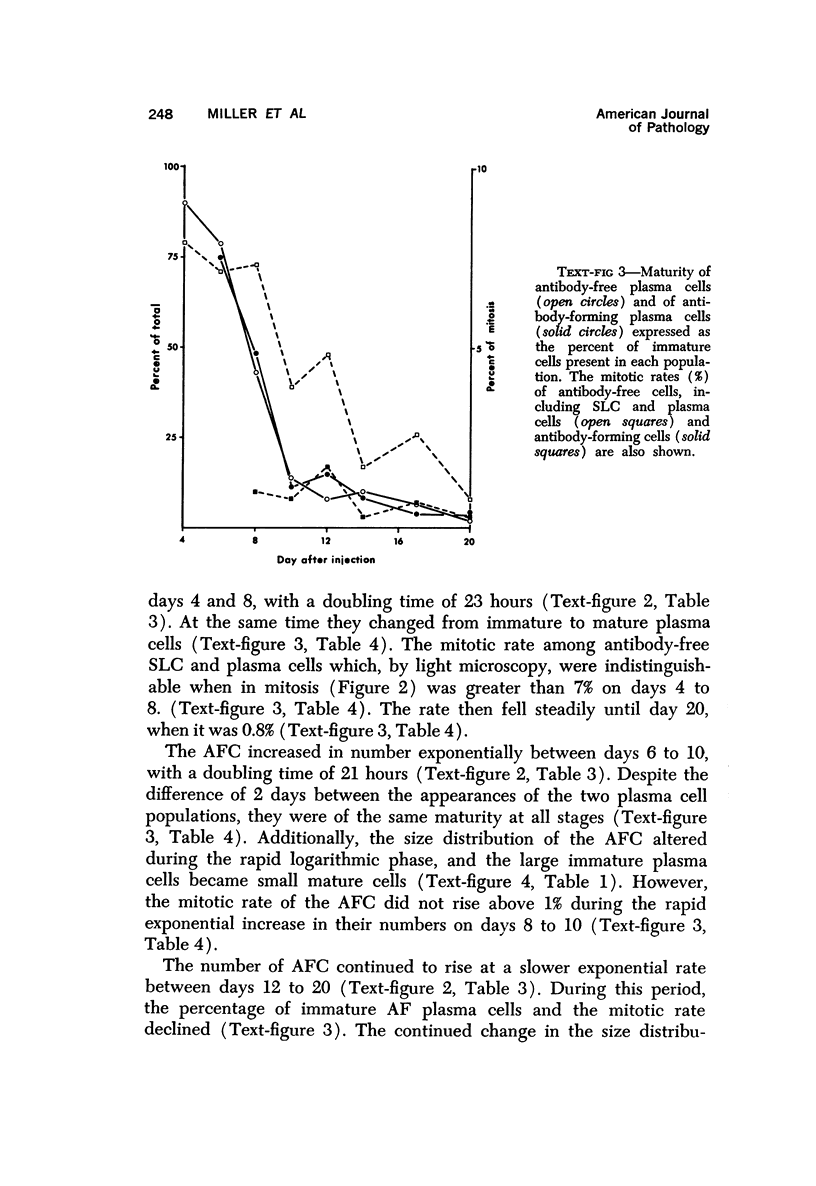
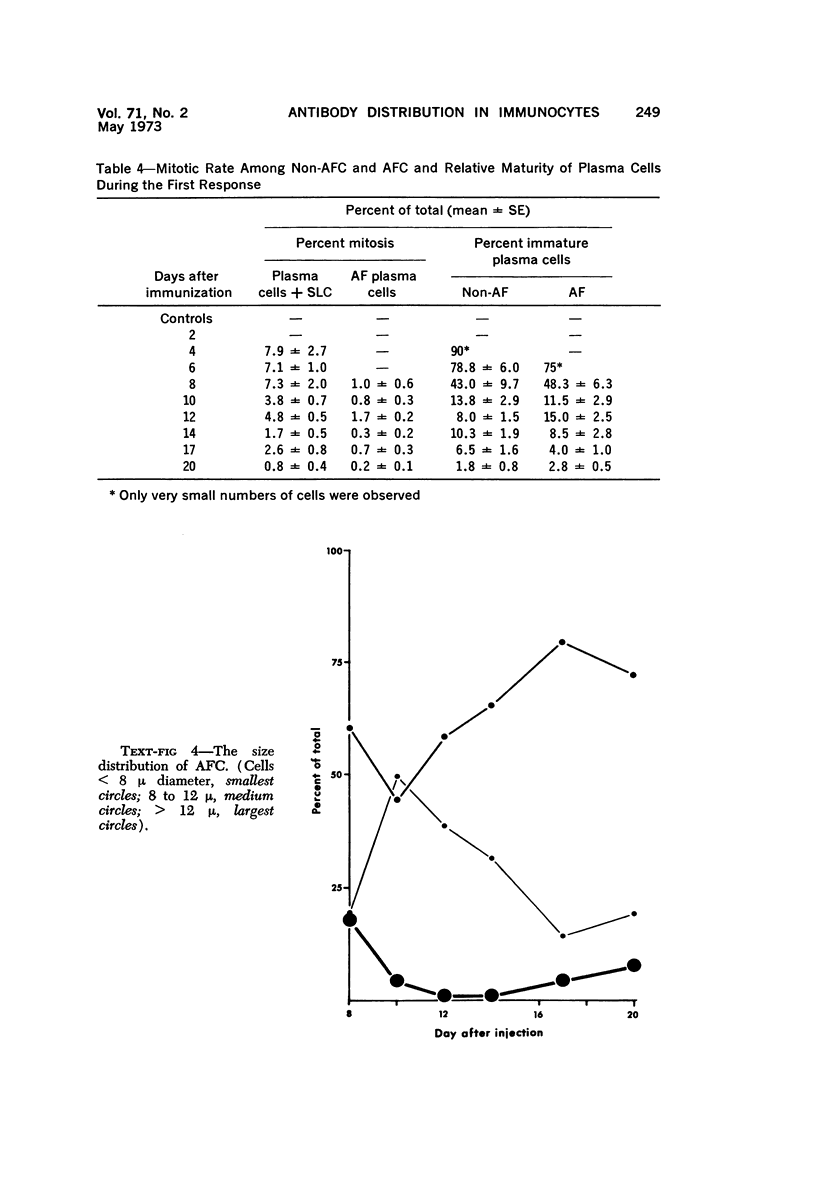
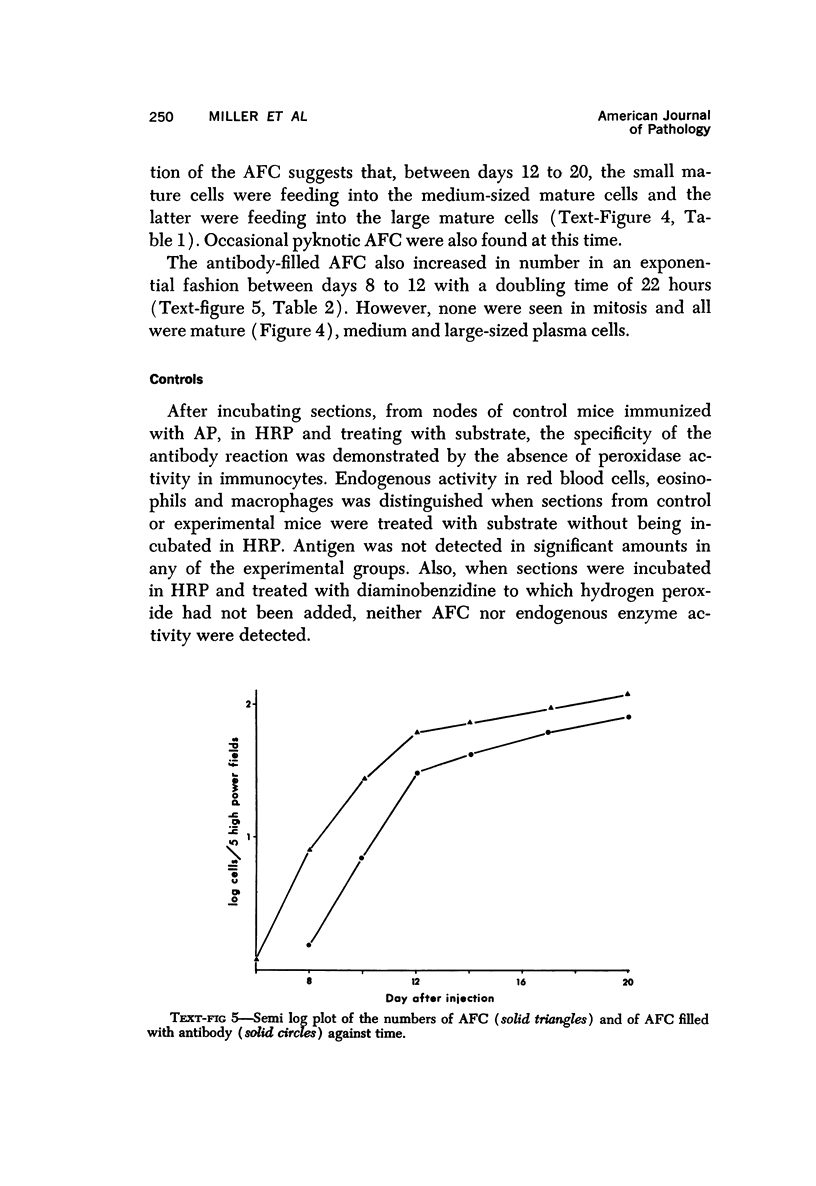
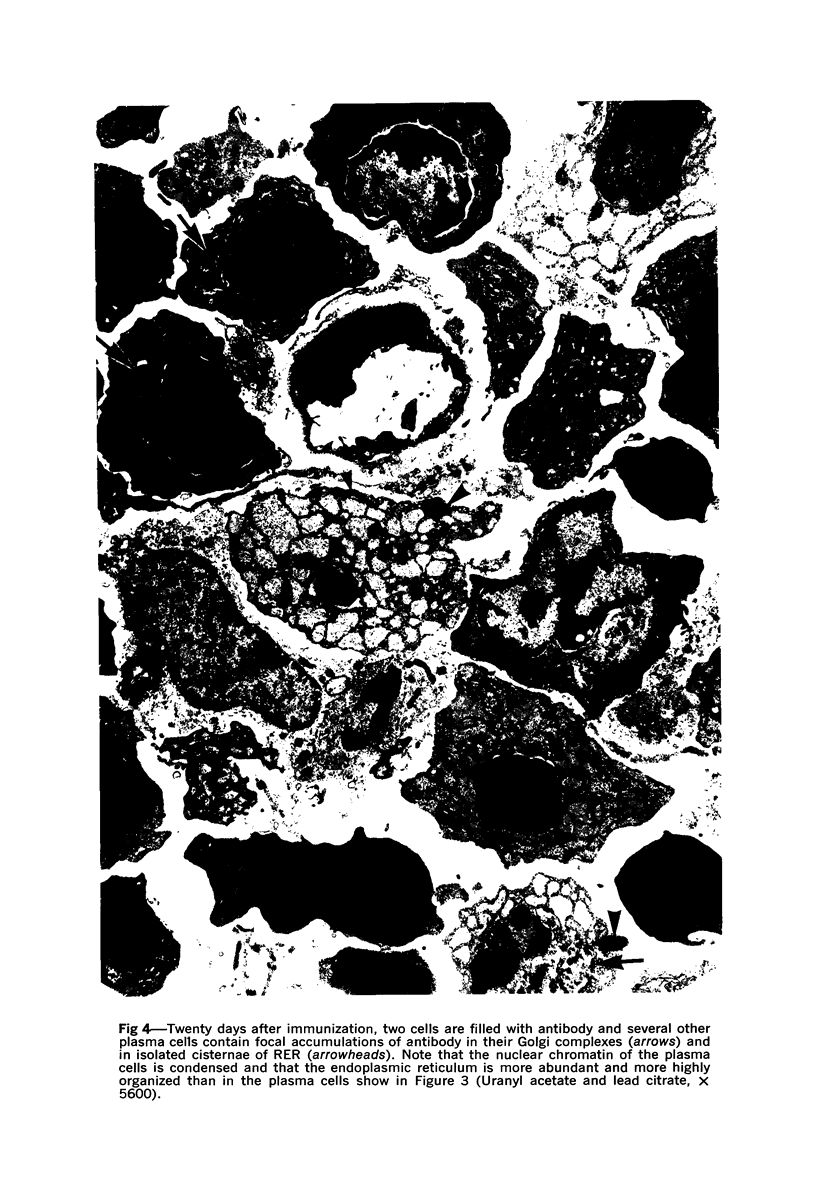
A new improved technic was used to follow the development of, and the intracellular antibody distribution in antiperoxidase antibody-forming cells (AFC) of the mouse popliteal lymph nodes responding to primary stimulation with horse-radish peroxidase (HRP). The first AFC were found 6 to 8 days after immunization and were all plasma cells. Antibody was concentrated in the Golgi complexes and in a few cisternae of rough endoplasmic reticulum. Subsequently, an increasing proportion of the AFC were filled with antibody, and with time the numbers of full cells and the intensity with which they stained increased. Kinetic studies of the cell changes in the lymph node medulla suggest that lymphoid cells proliferate, differentiate into plasma cells, and are then recruited as AFC. Furthermore, it was concluded that the changing intracellular distribution of antibody represents the gradual filling of the AFC with specific antibody.
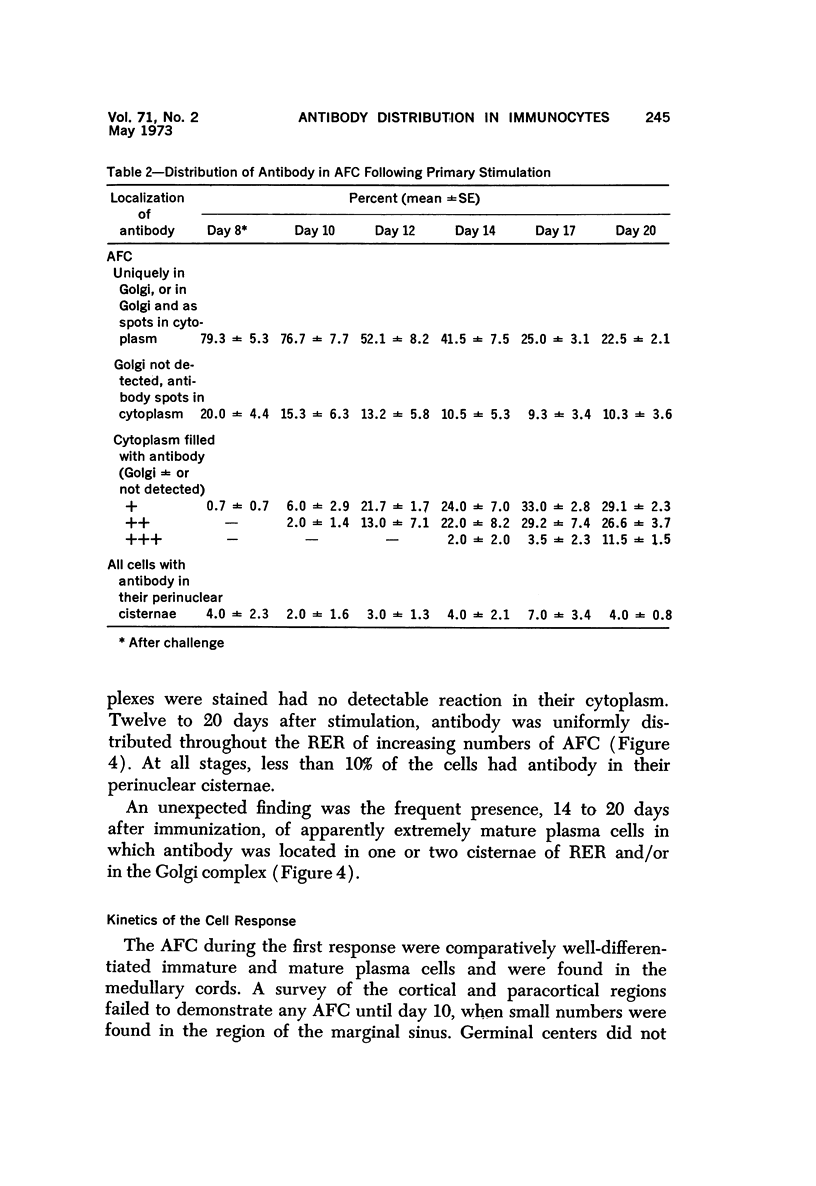
Full text
PDF




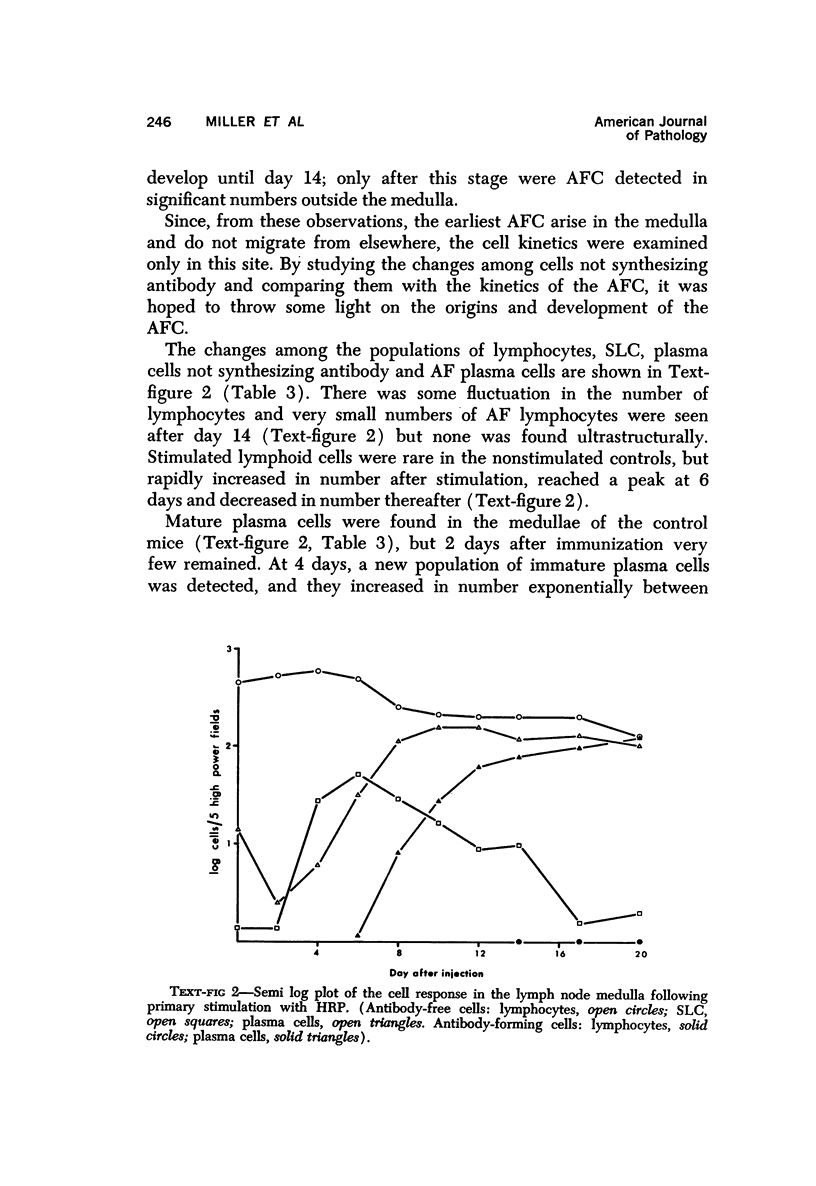
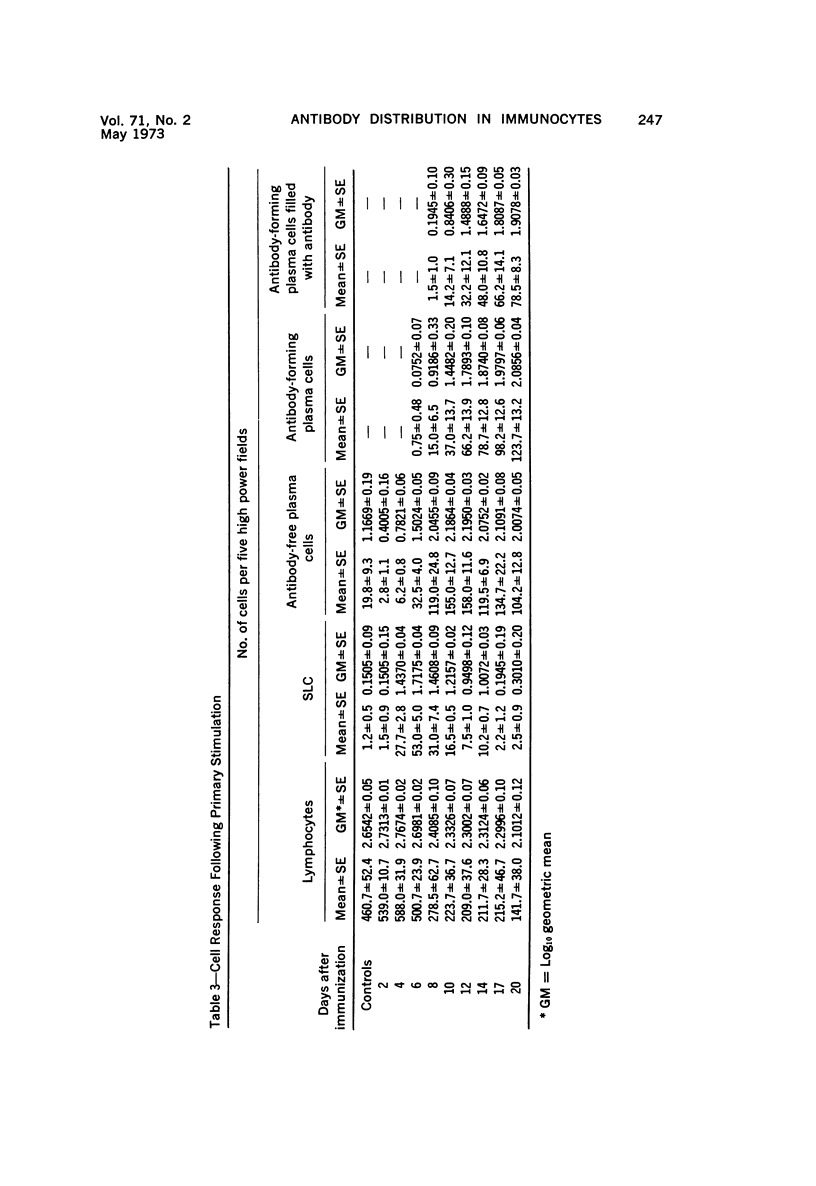








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S. Immunoenzyme techniques: enzymes as markers for the localization of antigens and antibodies. Int Rev Cytol. 1970;27:349–385. doi: 10.1016/s0074-7696(08)61250-4. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Kuhlmann W., Miller H. R., Leduc E. Two antibody-producing cell types in four species. Immunology. 1971 Jan;20(1):85–89. [PMC free article] [PubMed] [Google Scholar]
- Avrameas S., Leduc E. H. Detection of simultaneous antibody synthesis in plasma cells and specialized lymphocytes in rabbit lymph nodes. J Exp Med. 1970 Jun 1;131(6):1137–1168. doi: 10.1084/jem.131.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALFOUR B. M., COOPER E. H., ALPEN E. L. MORPHOLOGICAL AND KINETIC STUDIES ON ANTIBODY-PRODUCING CELLS IN RAT LYMPH NODES. Immunology. 1965 Mar;8:230–244. [PMC free article] [PubMed] [Google Scholar]
- Birbeck M. S., Hall J. G. Transformation, in vivo, of basophilic lymph cells into plasma cells. Nature. 1967 Apr 8;214(5084):183–185. doi: 10.1038/214183a0. [DOI] [PubMed] [Google Scholar]
- Bouteille M. Protein renewal in anti-peroxidase antibody forming cells. II. Combination of ultrastructural immuno-cytochemistry and autoradiography in "pulse-chase" experiments. Exp Cell Res. 1971 Nov;69(1):135–147. doi: 10.1016/0014-4827(71)90319-3. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Gudat F. G., Harris T. N., Harris S., Hummeler K. Studies on antibody-producing cells. 3. Identification of young plaque-forming cells by thymidine- 3 H labeling. J Exp Med. 1971 Nov 1;134(5):1155–1169. doi: 10.1084/jem.134.5.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. G., Morris B., Moreno G. D., Bessis M. C. The ultrastructure and function of the cells in lymph following antigenic stimulation. J Exp Med. 1967 Jan 1;125(1):91–110. doi: 10.1084/jem.125.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay J. B., Murphy M. J., Morris B., Bessis M. C. Quantitative studies on the proliferation and differentiation of antibody-forming cells in lymph. Am J Pathol. 1972 Jan;66(1):1–24. [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann W. D., Miller H. R. A comparative study of the techniques for ultrastructural localization of antienzyme antibodies. J Ultrastruct Res. 1971 May;35(3):370–385. doi: 10.1016/s0022-5320(71)80164-8. [DOI] [PubMed] [Google Scholar]
- Leduc E. H., Avrameas S., Bouteille M. Ultrastructural localization of antibody in differentiating plasma cells. J Exp Med. 1968 Jan 1;127(1):109–118. doi: 10.1084/jem.127.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER J. J., 3rd AN AUTORADIOGRAPHIC STUDY OF PLASMA CELL AND LYMPHOCYTE SURVIVAL IN RAT POPLITEAL LYMPH NODES. J Immunol. 1964 May;92:673–681. [PubMed] [Google Scholar]
- Makinodan T., Albright J. F. Proliferative and differentiative manifestations of cellular immune potential. Prog Allergy. 1967;10:1–36. [PubMed] [Google Scholar]
- Makinodan T., Sado T., Groves D. L., Price G. Growth patterns of antibody-forming cell populations. Curr Top Microbiol Immunol. 1969;49:80–113. doi: 10.1007/978-3-642-46166-8_2. [DOI] [PubMed] [Google Scholar]
- Miller H. R., Avrameas S., Ternynck T. Intracellular distribution of antibody in immunocytes responding to secondary challenge with horseradish peroxidase. Am J Pathol. 1973 May;71(2):261–278. [PMC free article] [PubMed] [Google Scholar]
- Miller H. R. Fixation and tissue preservation for antibody studies. Histochem J. 1972 Jul;4(4):305–320. doi: 10.1007/BF01005006. [DOI] [PubMed] [Google Scholar]
- Murphy M. J., Hay J. B., Morris B., Bessis M. C. Ultrastructural analysis of antibody synthesis in cells from lymph and lymph nodes. Am J Pathol. 1972 Jan;66(1):25–42. [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON K. C., JARETT L., FINKE E. H. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 1960 Nov;35:313–323. doi: 10.3109/10520296009114754. [DOI] [PubMed] [Google Scholar]
- Sordat B., Sordat M., Hess M. W., Stoner R. D., Cottier H. Specific antibody within lymphoid germinal center cells of mice after primary immunization with horseradish peroxidase: a light and electron microscopic study. J Exp Med. 1970 Jan 1;131(1):77–91. doi: 10.1084/jem.131.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus W. Location of antibody to horseradish peroxidase in popliteal lymph nodes of rabbits during the primary and early secondary response. J Histochem Cytochem. 1970 Feb;18(2):120–130. doi: 10.1177/18.2.120. [DOI] [PubMed] [Google Scholar]