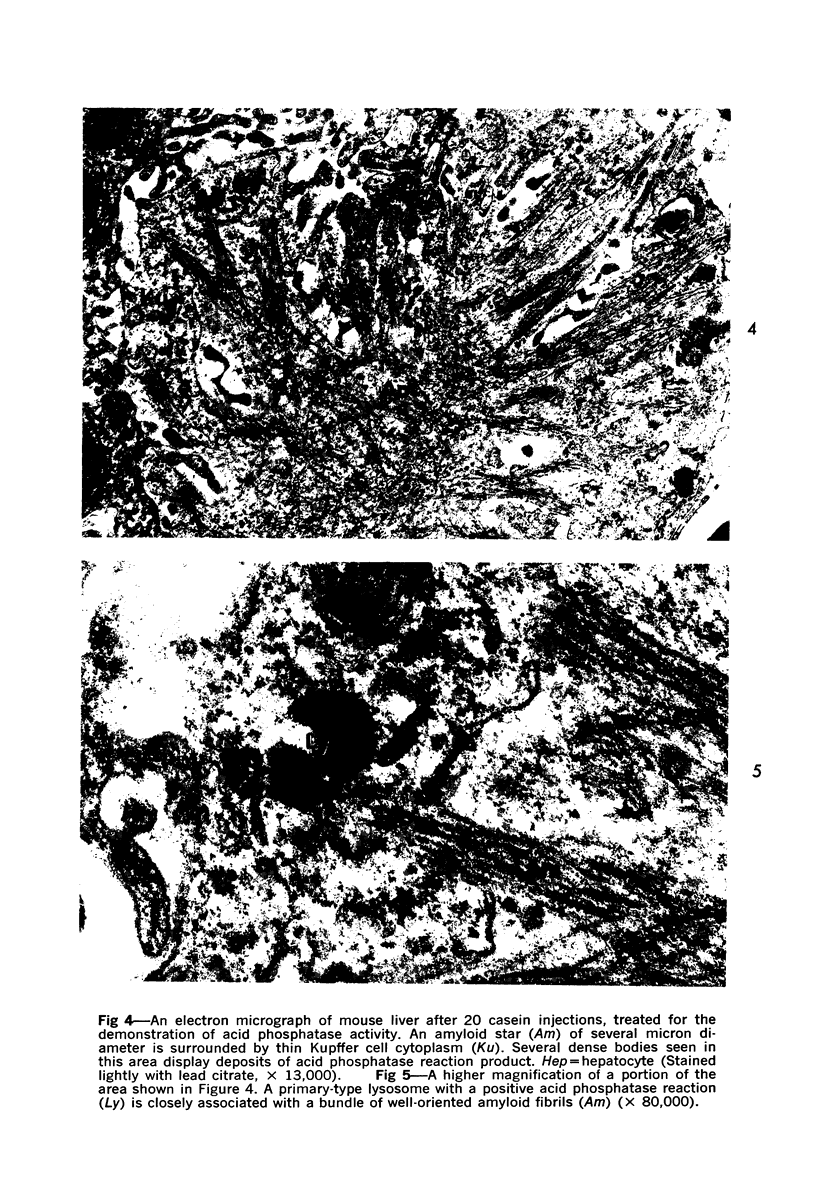
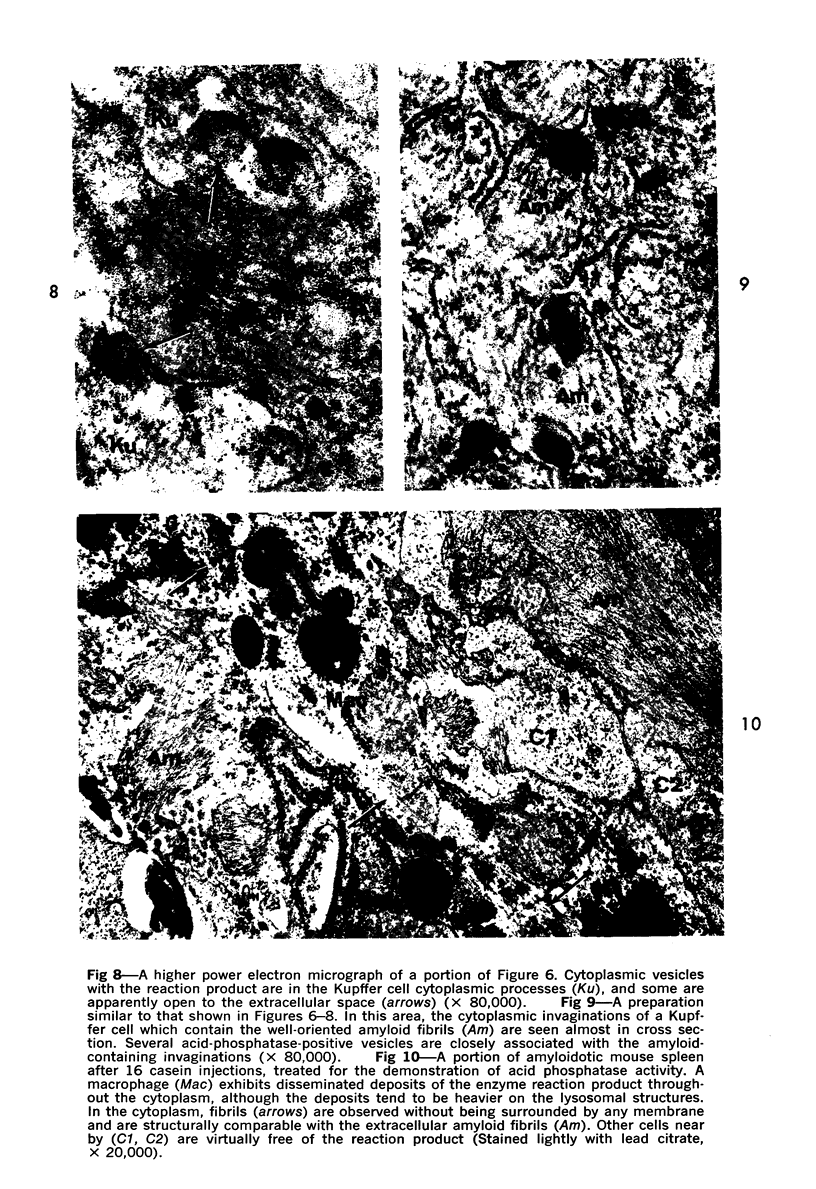
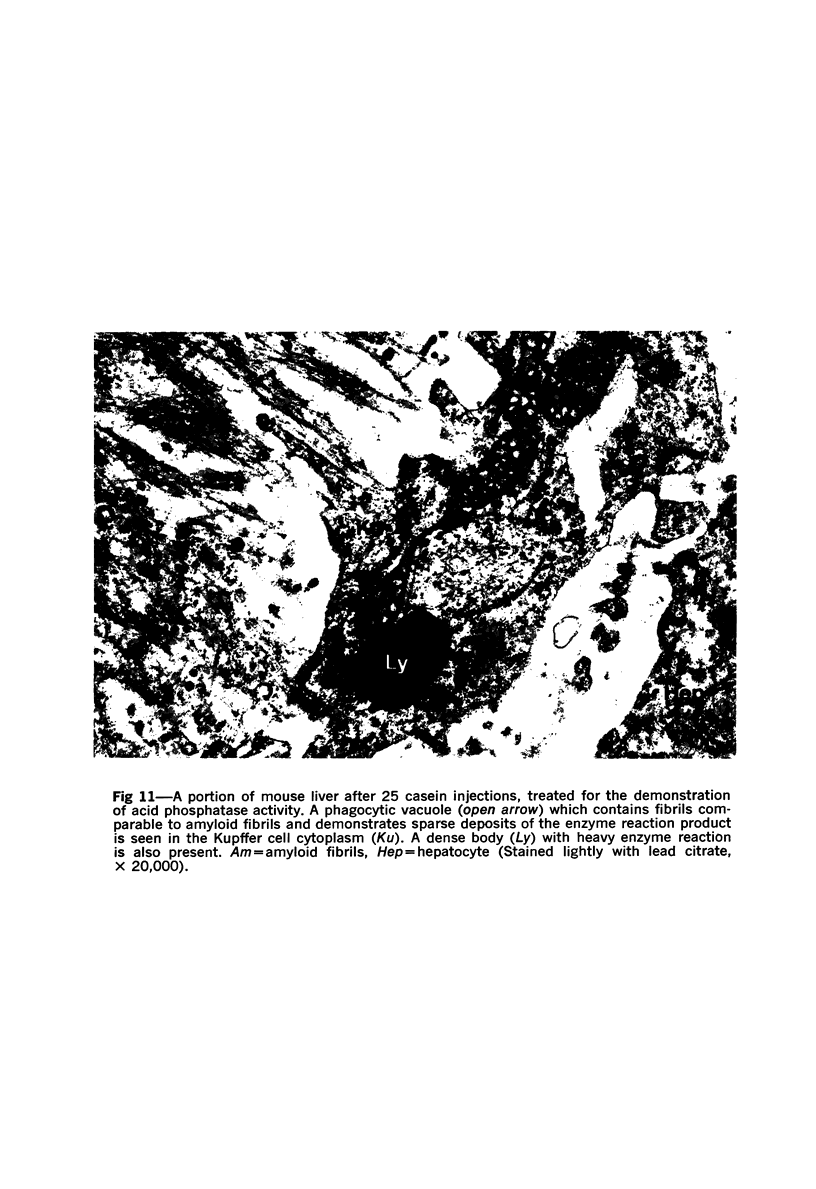
Abstract
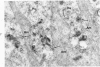
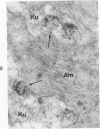
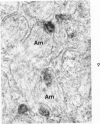
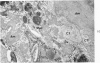
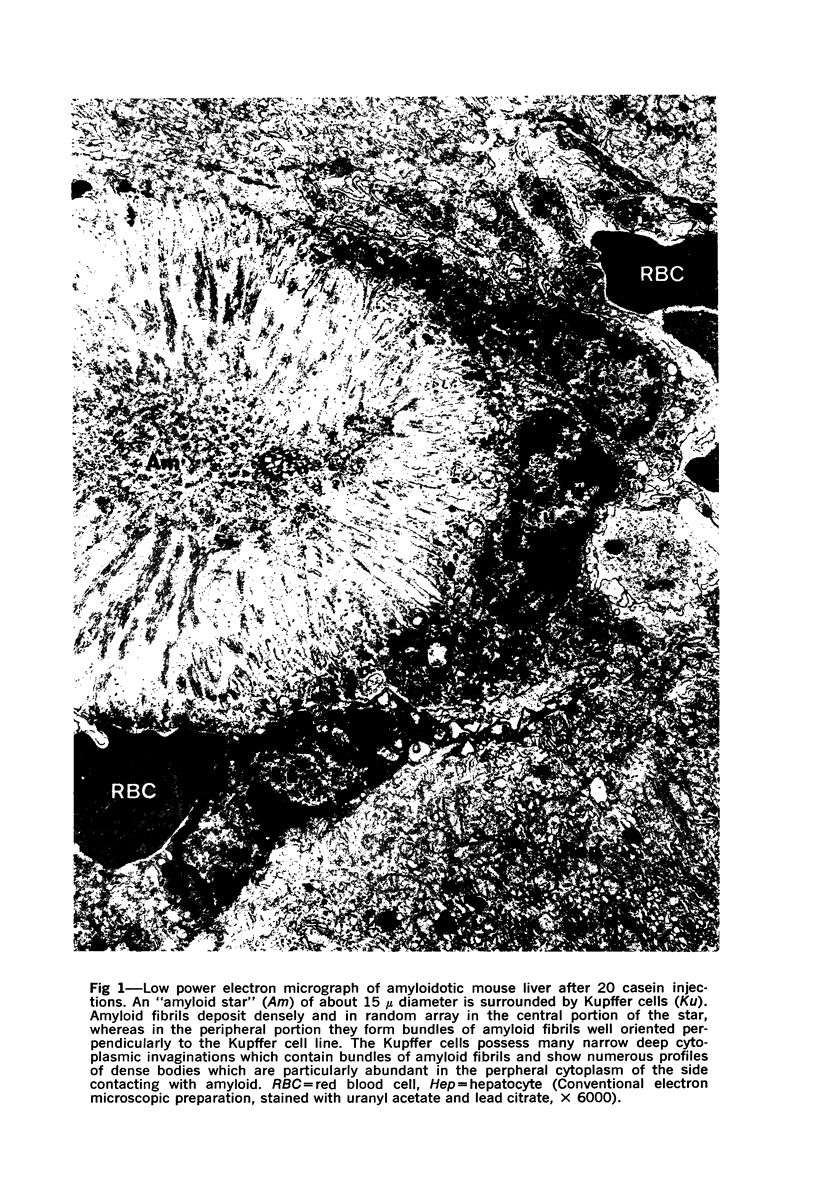
On the basis of recent morpholgic and biochemical studies which suggested the possible involvement of reticuloendothelial (RE) cells and proteolytic enzymes in amyloidogenesis, the present study was undertaken to examine the ultrastructural interrelationship between lysosomes and amyloid fibrils at the sites of very early amyloid deposition. In the spleen, liver and kidney of the experimental mouse model, foci of amyloid deposits were closely associated with the RE cells. The lysosomal enzyme activity, as marked by cytochemical demonstration of acid glycerophosphatase activity, was localized in the primary type lysosomes (as defined by their electron microscopic appearance), in the Golgi complexes, in the small cytoplasmic vesicles and occasionally widespread in the cytoplasm. They showed an intimate relationship to the amyloid fibrils. The findings were interpreted as favoring the hypothesis that the hydrolases play a role in amyloid fibril formation. The enzyme activity was also demonstrated in the secondary type lysosomes which occasionally contained amyloid fibrils that appeared to be phagocytized.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bari W. A., Pettengill O. S., Sorenson G. D. Electron microscopy and electron microscopic autoradiography of splenic cell cultures from mice with amyloidosis. Lab Invest. 1969 Mar;20(3):234–242. [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N., Hermodson M. A., Ericsson L. H. The major proteins of human and monkey amyloid substance: Common properties including unusual N-terminal amino acid sequences. FEBS Lett. 1971 Dec 1;19(2):169–173. doi: 10.1016/0014-5793(71)80506-9. [DOI] [PubMed] [Google Scholar]
- Carr I. The fine structure of the mammalian lymphoreticular system. Int Rev Cytol. 1970;27:283–348. doi: 10.1016/s0074-7696(08)61249-8. [DOI] [PubMed] [Google Scholar]
- Cohen A. S., Gross E., Shirahama T. The light and electron microscopic autoradiographic demonstration of local amyloid formation in spleen explants. Am J Pathol. 1965 Dec;47(6):1079–1111. [PMC free article] [PubMed] [Google Scholar]
- Cohen A. S., Shirahama T. Animal model for human disease: spontaneous and induced amyloidosis. Am J Pathol. 1972 Aug;68(2):441–444. [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A. The structure and function of monocytes and macrophages. Adv Immunol. 1968;9:163–214. doi: 10.1016/s0065-2776(08)60443-5. [DOI] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Ein D., Kimura S., Glenner G. G. An amyloid fibril protein of unknown origin: partial amino-acid sequence analysis. Biochem Biophys Res Commun. 1972 Jan 31;46(2):498–500. doi: 10.1016/s0006-291x(72)80166-9. [DOI] [PubMed] [Google Scholar]
- Franklin E. C., Pras M., Levin M., Frangione B. The partial amino acid sequence of the major low molecular weight component of two human amyloid fibrils. FEBS Lett. 1972 Apr 15;22(1):121–123. doi: 10.1016/0014-5793(72)80235-7. [DOI] [PubMed] [Google Scholar]
- GUEFT B., GHIDONI J. J. THE SITE OF FORMATION AND ULTRASTRUCTURE OF AMYLOID. Am J Pathol. 1963 Nov;43:837–854. [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G., Ein D., Eanes E. D., Bladen H. A., Terry W., Page D. L. Creation of "amyloid" fibrils from Bence Jones proteins in vitro. Science. 1971 Nov 12;174(4010):712–714. doi: 10.1126/science.174.4010.712. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Terry W., Harada M., Isersky C., Page D. Amyloid fibril proteins: proof of homology with immunoglobulin light chains by sequence analyses. Science. 1971 Jun 11;172(3988):1150–1151. doi: 10.1126/science.172.3988.1150. [DOI] [PubMed] [Google Scholar]
- Husby G., Sletten K., Michaelsen T. E., Natvig J. B. Alternative, non-immunoglobulin origin of amyloid fibrils. Nat New Biol. 1972 Aug 9;238(84):187–187. doi: 10.1038/newbio238187a0. [DOI] [PubMed] [Google Scholar]
- Katchburian E., Holt S. J. Role of lysosomes in amelogenesis. Nature. 1969 Sep 27;223(5213):1367–1368. doi: 10.1038/2231367a0. [DOI] [PubMed] [Google Scholar]
- Kazimierczak J. Cytochemical study of casein-induced and nitrogen mustard accelerated amyloidosis in mice. Acta Pathol Microbiol Scand. 1969;77(2):201–217. doi: 10.1111/j.1699-0463.1969.tb04225.x. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. Y., Yam L. T., Lam K. W. Acid phosphatase isoenzyme in human leukocytes in normal and pathologic conditions. J Histochem Cytochem. 1970 Jul;18(7):473–481. doi: 10.1177/18.7.473. [DOI] [PubMed] [Google Scholar]
- Lin C. W., Fishman W. H. Microsomal and lysosomal acid phosphatase isoenzymes of mouse kidney. Characterization and separation. J Histochem Cytochem. 1972 Jul;20(7):487–498. doi: 10.1177/20.7.487. [DOI] [PubMed] [Google Scholar]
- MILLER F., PALADE G. E. LYTIC ACTIVITIES IN RENAL PROTEIN ABSORPTION DROPLETS. AN ELECTRON MICROSCOPICAL CYTOCHEMICAL STUDY. J Cell Biol. 1964 Dec;23:519–552. doi: 10.1083/jcb.23.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi V. Lysosomal and non-lysosomal localization of acid hydrolases in animal cells. Biochem J. 1969 Mar;111(5):25P–26P. doi: 10.1042/bj1110025p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVIKOFF A. B., ESSNER E., QUINTANA N. GOLGI APPARATUS AND LYSOSOMES. Fed Proc. 1964 Sep-Oct;23:1010–1022. [PubMed] [Google Scholar]
- North R. J. The localization by electron microscopy of acid phosphatase activity in guinea pig macrophages. J Ultrastruct Res. 1966 Sep;16(1):96–108. doi: 10.1016/s0022-5320(66)80025-4. [DOI] [PubMed] [Google Scholar]
- SMITH R. E., FARQUHAR M. G. PREPARATION OF THICK SECTIONS FOR CYTOCHEMISTRY AND ELECTRON MICROSCOPY BY A NON-FREEZING TECHNIQUE. Nature. 1963 Nov 16;200:691–691. doi: 10.1038/200691a0. [DOI] [PubMed] [Google Scholar]
- Scarpelli D. G., Kanczak N. M. Ultrastructural cytochemistry: principles, limitations, and applications. Int Rev Exp Pathol. 1965;4:55–126. [PubMed] [Google Scholar]
- Shirahama T., Benson M. D., Cohen A. S., Tanaka A. Fibrillar assemblage of variable segments of immunoglobulin light chains: an electron microscopic study. J Immunol. 1973 Jan;110(1):21–30. [PubMed] [Google Scholar]
- Shirahama T., Cohen A. S. Lysosomal breakdown of amyloid fibrils by macrophages. Am J Pathol. 1971 Jun;63(3):463–486. [PMC free article] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock A., Leblond C. P. Elaboration of the matrix glycoprotein of enamel by the secretory ameloblasts of the rat incisor as revealed by radioautography after galactose- 3 H injection. J Cell Biol. 1971 Oct;51(1):26–51. doi: 10.1083/jcb.51.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Dukor P. The role of lysosomes in immune responses. Adv Immunol. 1970;12:283–331. doi: 10.1016/s0065-2776(08)60172-8. [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D., Franklin E. C. Intracellular localization of human amyloid by fluorescence and electron microscopy. Am J Pathol. 1970 Apr;59(1):23–41. [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D. Immunophagocytosis of human amyloid fibrils by leukocytes. J Ultrastruct Res. 1970 Aug;32(3):247–257. doi: 10.1016/s0022-5320(70)80005-3. [DOI] [PubMed] [Google Scholar]