Abstract
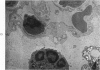
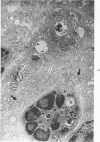
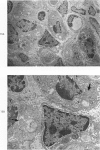
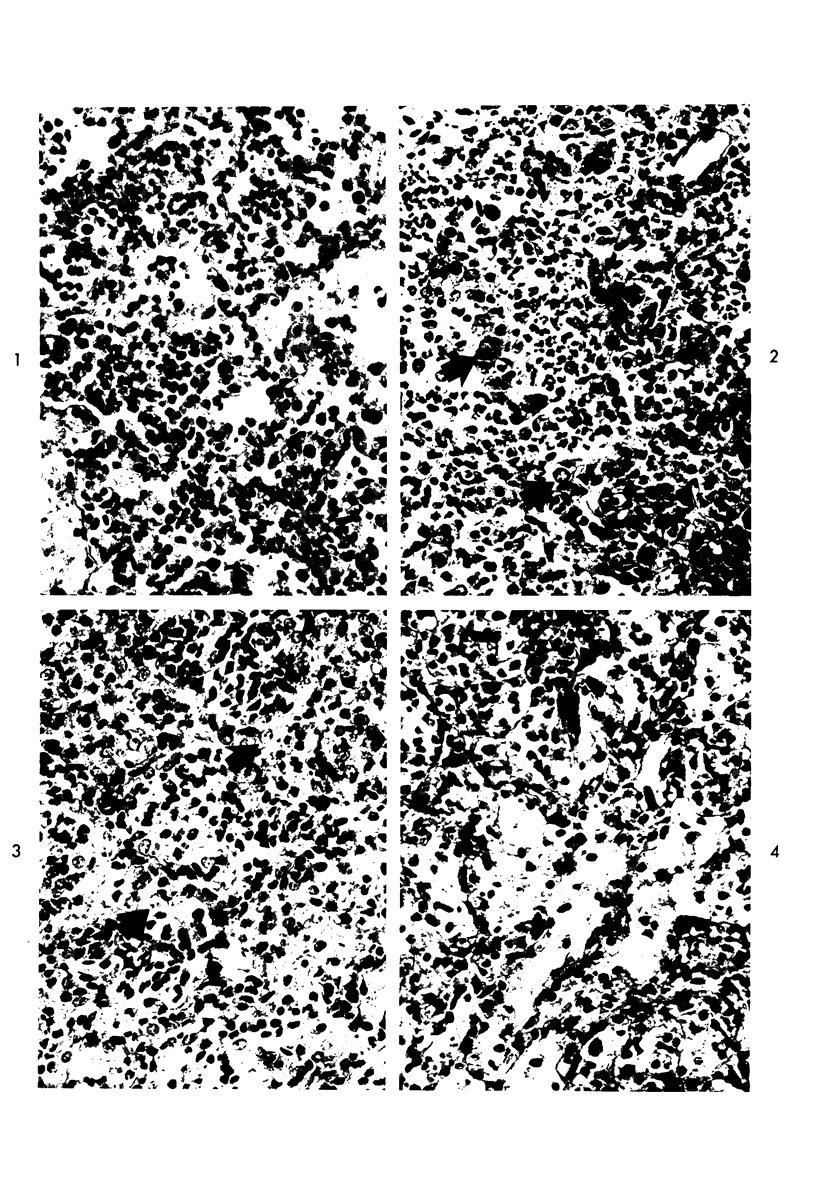
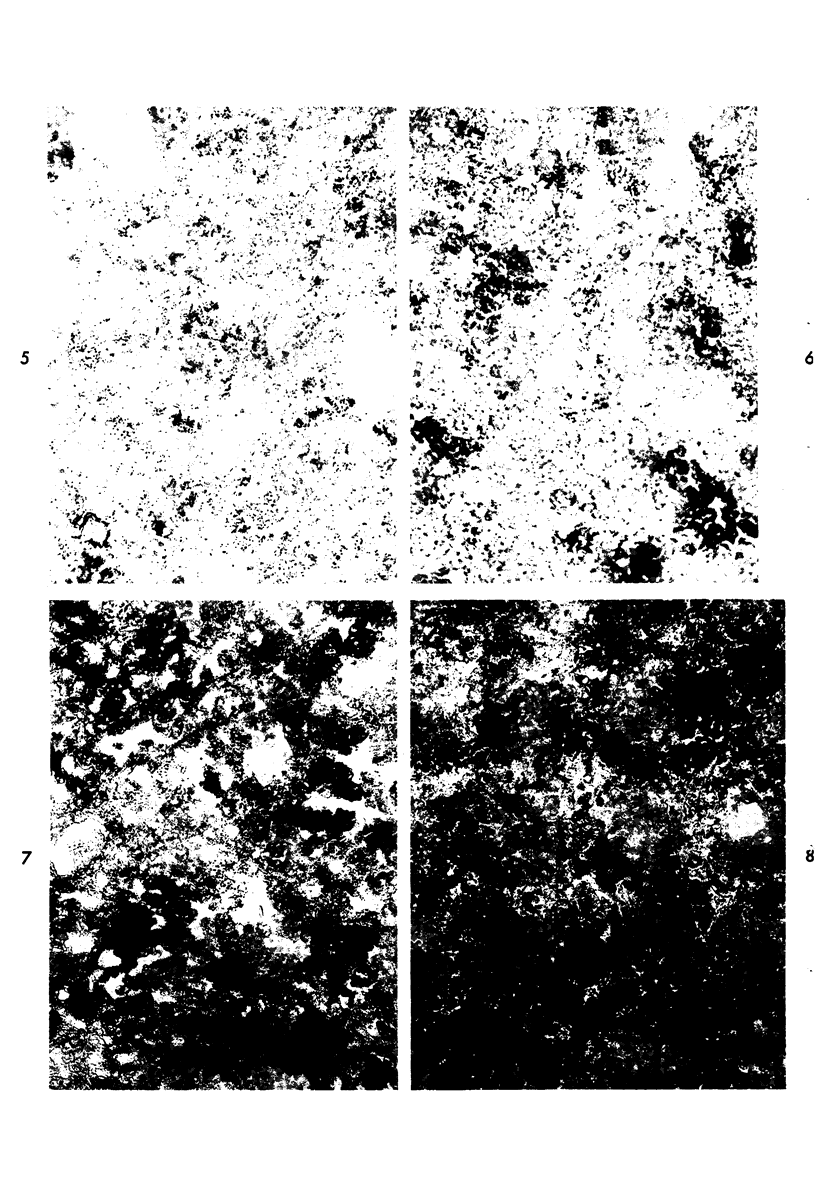
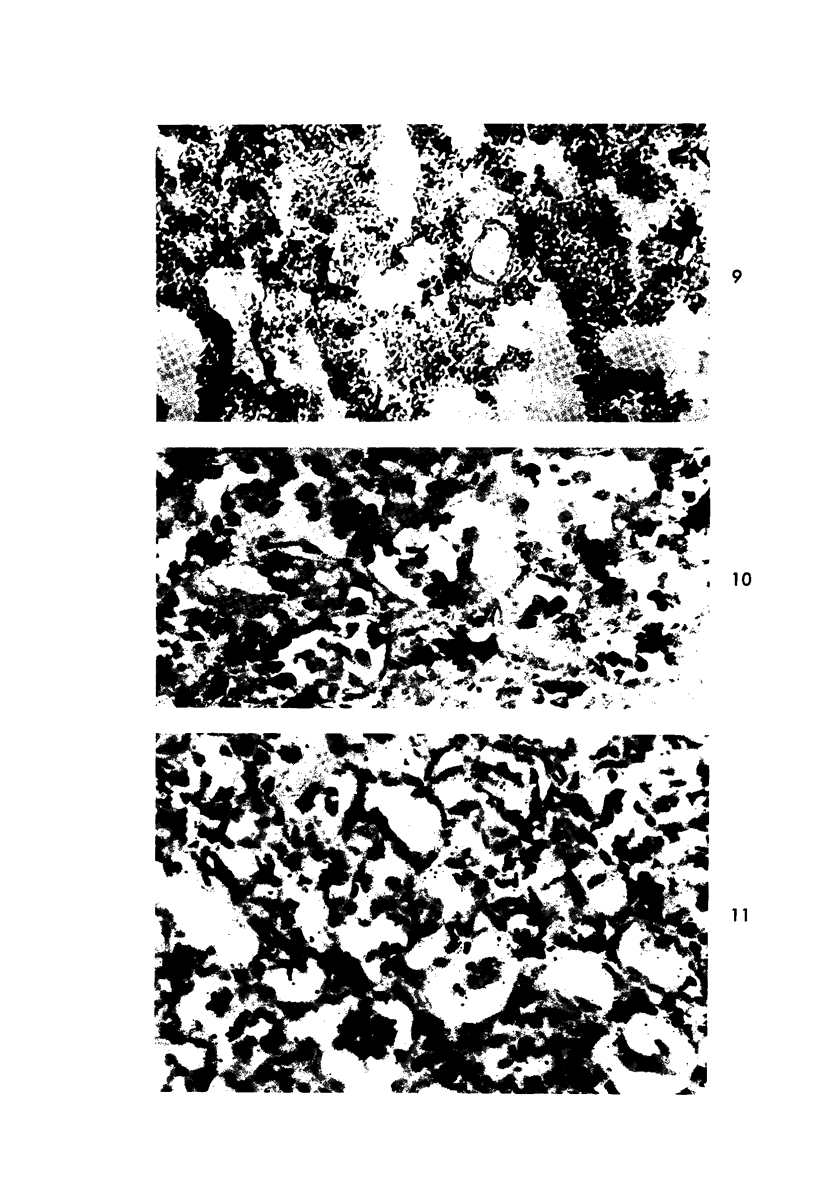
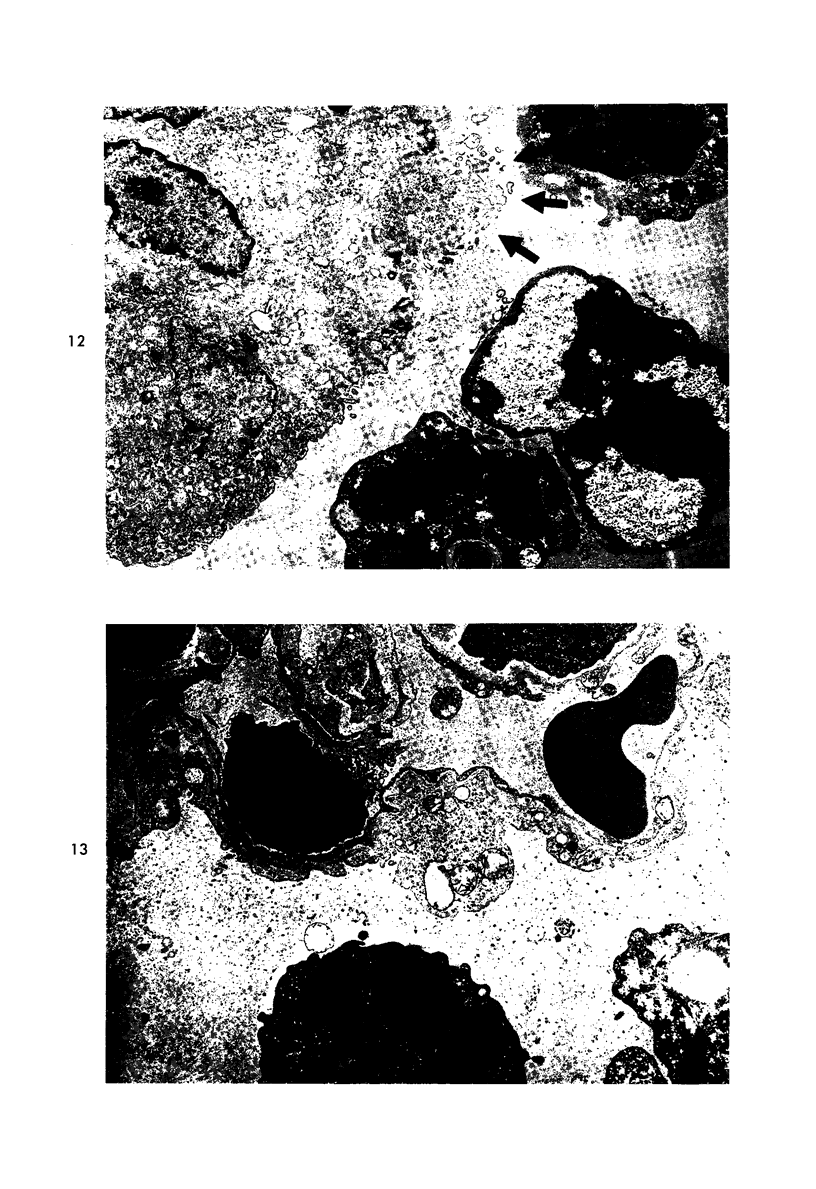
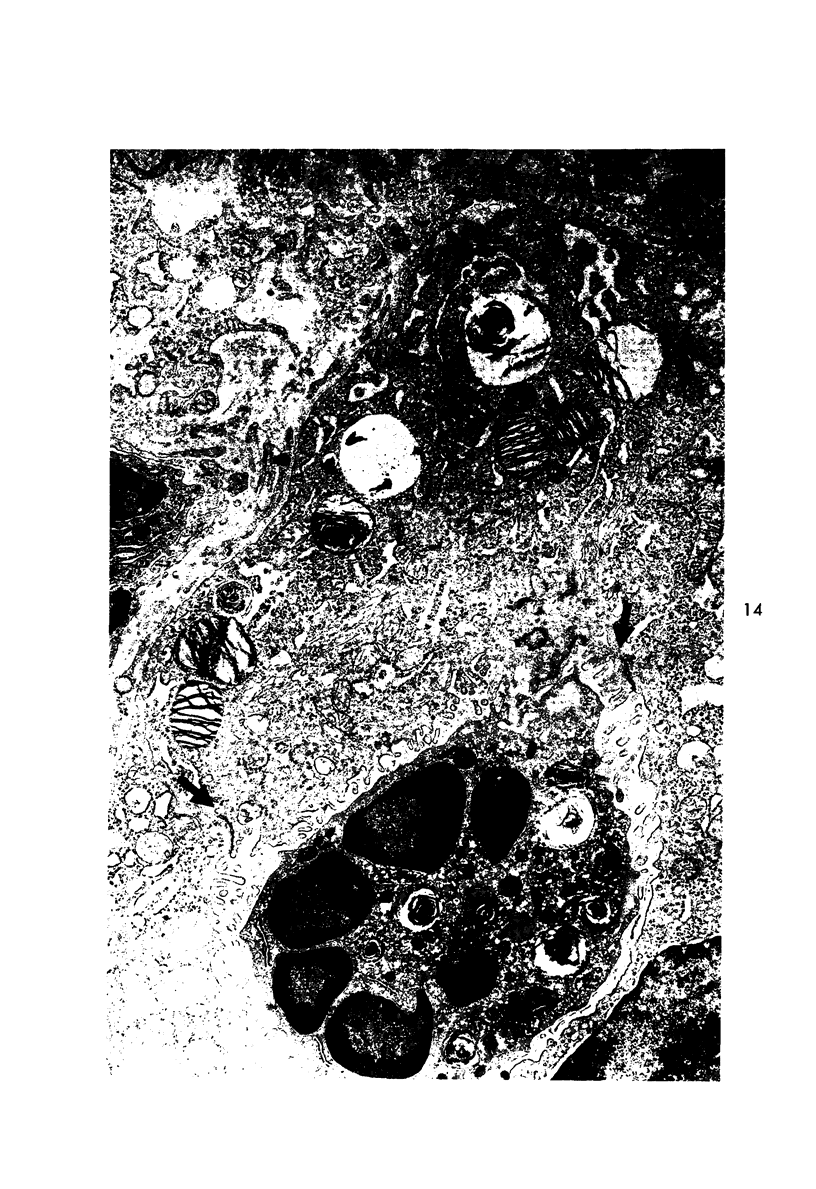
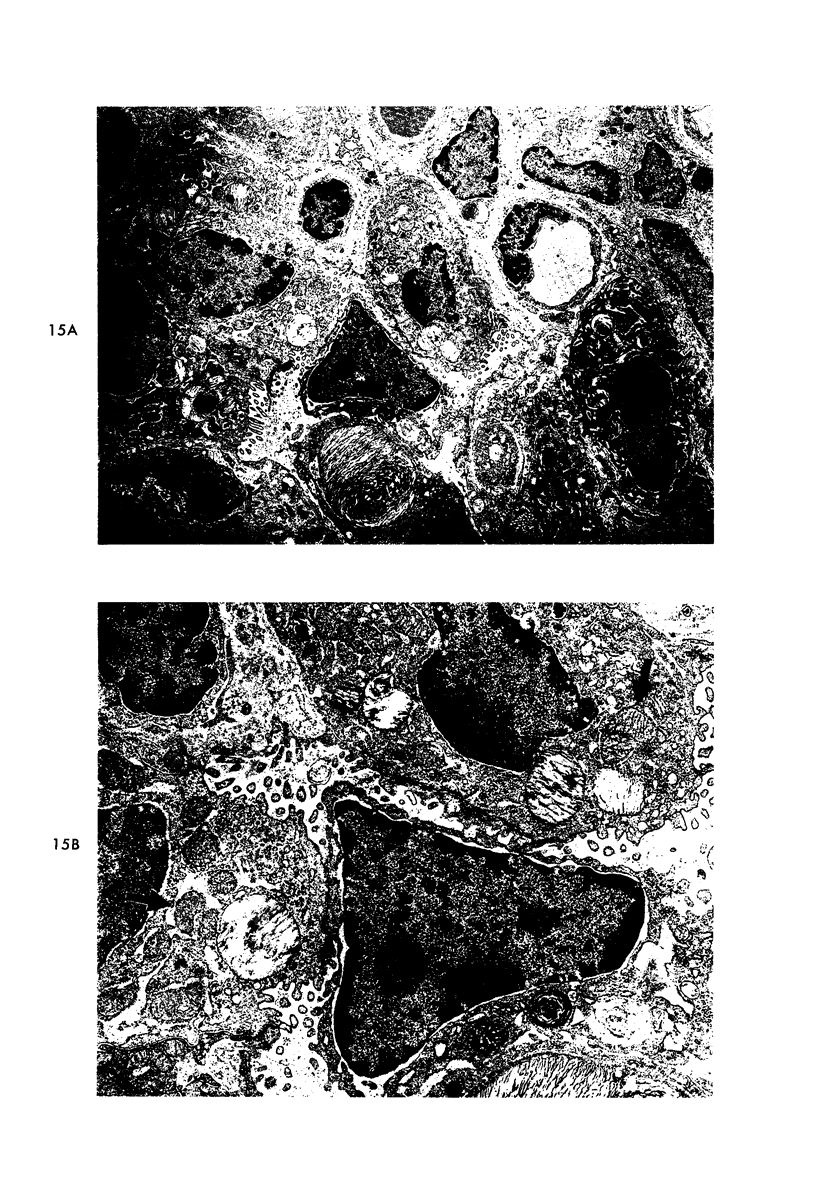
Proteus infection in the rat produces an acute bacterial pneumonia which resolves without necrosis or significant organization within 2 weeks. Ultrastructural changes included widespread damage to type 2 cells and endothelial cells and rapid proliferation of the alveolar epithelial cells, which were the predominant site of labeling with tritiated thymidine. Histochemical staining for lysosomal enzymes showed an initial reduction in type 2 cell reactivity. The majority of proliferating epithelial cells were also unreactive until the normal pattern of staining and morphology returned at 8 to 12 days after infection. The acute inflammatory exudate was reactive, but there was only minimal to moderate staining in the subsequent clusters of alveolar macrophages. These data suggest that resolution with the preservation of the normal architecture of the peripheral airspaces may be correlated with superficial injury and limited reactivity for digestive enzymes.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURSTONE M. S. Histochemical comparison of naphthol AS-phosphates for the demonstration of phosphatases. J Natl Cancer Inst. 1958 Mar;20(3):601–615. [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. The pulmonary interstitial cell as immediate precursor of the alveolar macrophage. Am J Pathol. 1972 Sep;68(3):521–537. [PMC free article] [PubMed] [Google Scholar]
- Brennan P. C., Fritz T. E., Flynn R. J. Role of Pasteurella pneumotropica and Mycoplasma pulmonis in murine pneumonia. J Bacteriol. 1969 Jan;97(1):337–349. doi: 10.1128/jb.97.1.337-349.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington C. B., Green T. J. Granular pneumocytes in early repair of diffuse alveolar injury. Arch Intern Med. 1970 Sep;126(3):464–465. [PubMed] [Google Scholar]
- DANNENBERG A. M., Jr, BURSTONE M. S., WALTER P. C., KINSLEY J. W. A histochemical study of phagocytic and enzymatic functions of rabbit mononuclear and polymorphonuclear exudate cells and alveolar macrophages. I. Survey and quantitation of enzymes, and states of cellular activation. J Cell Biol. 1963 Jun;17:465–486. doi: 10.1083/jcb.17.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoust R. Histochemical localization of enzyme activities by substrate film methods: ribonucleases, deoxyribonucleases, proteases, amylase, and hyaluronidase. Int Rev Cytol. 1965;18:191–221. doi: 10.1016/s0074-7696(08)60554-9. [DOI] [PubMed] [Google Scholar]
- Esterly J. R., Standen A. C., Pearson B. Hydrolytic enzymes in the neonatal rat lung. Am Rev Respir Dis. 1969 Sep;100(3):321–326. doi: 10.1164/arrd.1969.100.3.321. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Cabral L. J., Stephens R. J., Freeman G. Renewal of alveolar epithelium in the rat following exposure to NO2. Am J Pathol. 1973 Feb;70(2):175–198. [PMC free article] [PubMed] [Google Scholar]
- Faulkner C. S., 2nd, Esterly J. R. Ultrastructural changes in the alveolar epithelium in response to Freund's adjuvant. Am J Pathol. 1971 Sep;64(3):559–566. [PMC free article] [PubMed] [Google Scholar]
- Golfischer S., Kikkawa Y., Hoffman L. The demonstration of acid hydrolase activities in the inclusion bodies of type II alveolar cells and other lysosomes in the rabbit lung. J Histochem Cytochem. 1968 Feb;16(2):102–109. doi: 10.1177/16.2.102. [DOI] [PubMed] [Google Scholar]
- NACHLAS M. M., TSOU K. C., DE SOUZA E., CHENG C. S., SELIGMAN A. M. Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole. J Histochem Cytochem. 1957 Jul;5(4):420–436. doi: 10.1177/5.4.420. [DOI] [PubMed] [Google Scholar]
- PEARSON B., GROSE F. Further histochemical studies of esterases by 5-bromoindoxyl acetate. I. Reference to nonspecific and nonenzymatic hydrolysis. AMA Arch Pathol. 1959 Mar;67(3):324–332. [PubMed] [Google Scholar]
- PEARSON B., WOLF P. L., VAZQUEZ J. A COMPARATIVE STUDY OF A SERIES OF NEW INDOLYL COMPOUNDS TO LOCALIZE BETA-GALACTOSIDASE IN TISSUES. Lab Invest. 1963 Dec;12:1249–1259. [PubMed] [Google Scholar]
- PUGH D., WALKER P. G. The localization of N-acetyl-beta-glucosaminidase in tissues. J Histochem Cytochem. 1961 May;9:242–250. doi: 10.1177/9.3.242. [DOI] [PubMed] [Google Scholar]
- Pearson B., Standen A. C., Esterly J. R. Histochemical beta-glucuronidase distribution in mammalian tissue as detected by 5-bromo-4-chloroindol-3-yl-beta-D-glucopyruroniside. Lab Invest. 1967 Aug;17(2):217–224. [PubMed] [Google Scholar]
- Robertson O. H., Coggeshall L. T., Terrell E. E. EXPERIMENTAL PNEUMOCOCCUS LOBAR PNEUMONIA IN THE DOG: II. Pathology. J Clin Invest. 1933 Mar;12(2):433–466. doi: 10.1172/JCI100511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO K., SUTER E. LYSOSOMAL ACID HYDROLASES IN MICE INFECTED WITH BCG. J Exp Med. 1965 May 1;121:727–738. doi: 10.1084/jem.121.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin S P. A morphologic and cytochemical study on the great alveolar cell. J Histochem Cytochem. 1966 Dec;14(12):884–897. doi: 10.1177/14.12.884. [DOI] [PubMed] [Google Scholar]
- Yamamoto E., Wittner M., Rosenbaum R. M. Resistance and susceptibility to oxygen toxicity by cell types of the gas-blood barrier of the rat lung. Am J Pathol. 1970 Jun;59(3):409–436. [PMC free article] [PubMed] [Google Scholar]