Abstract
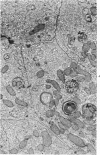
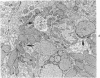
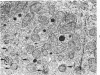
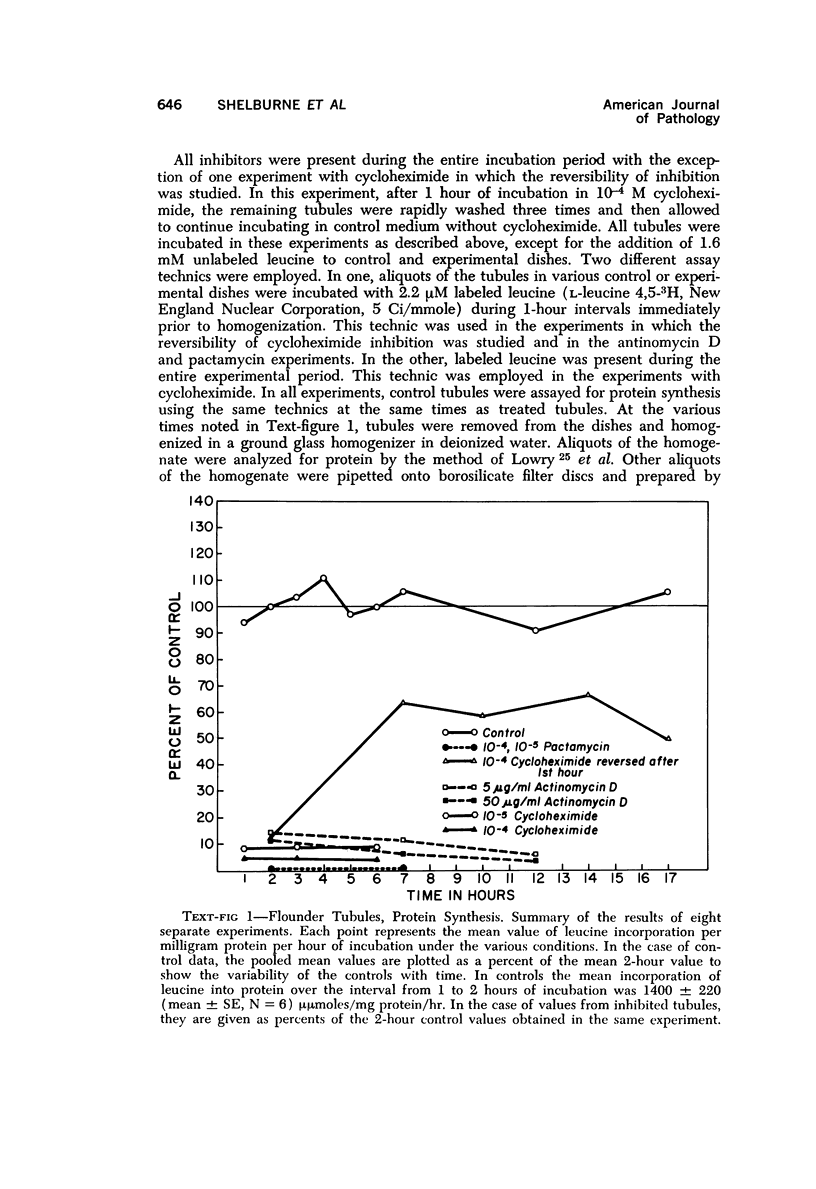
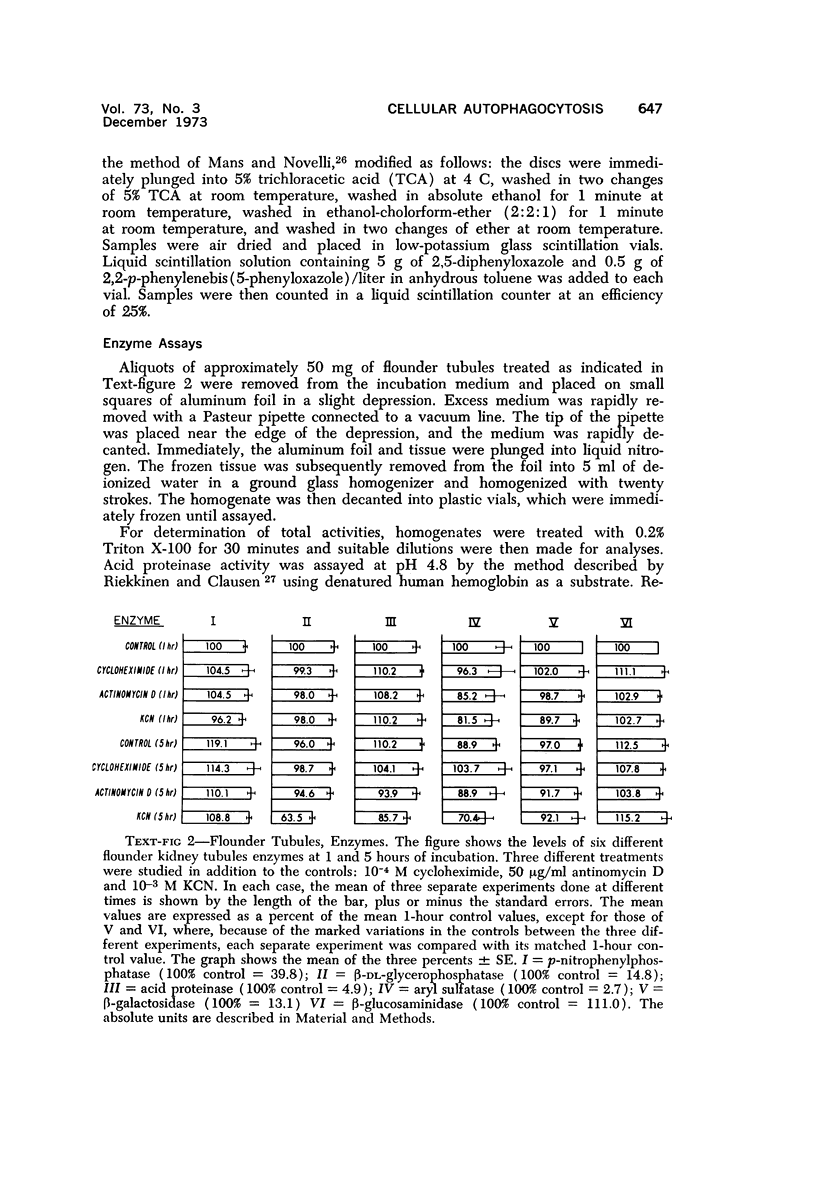
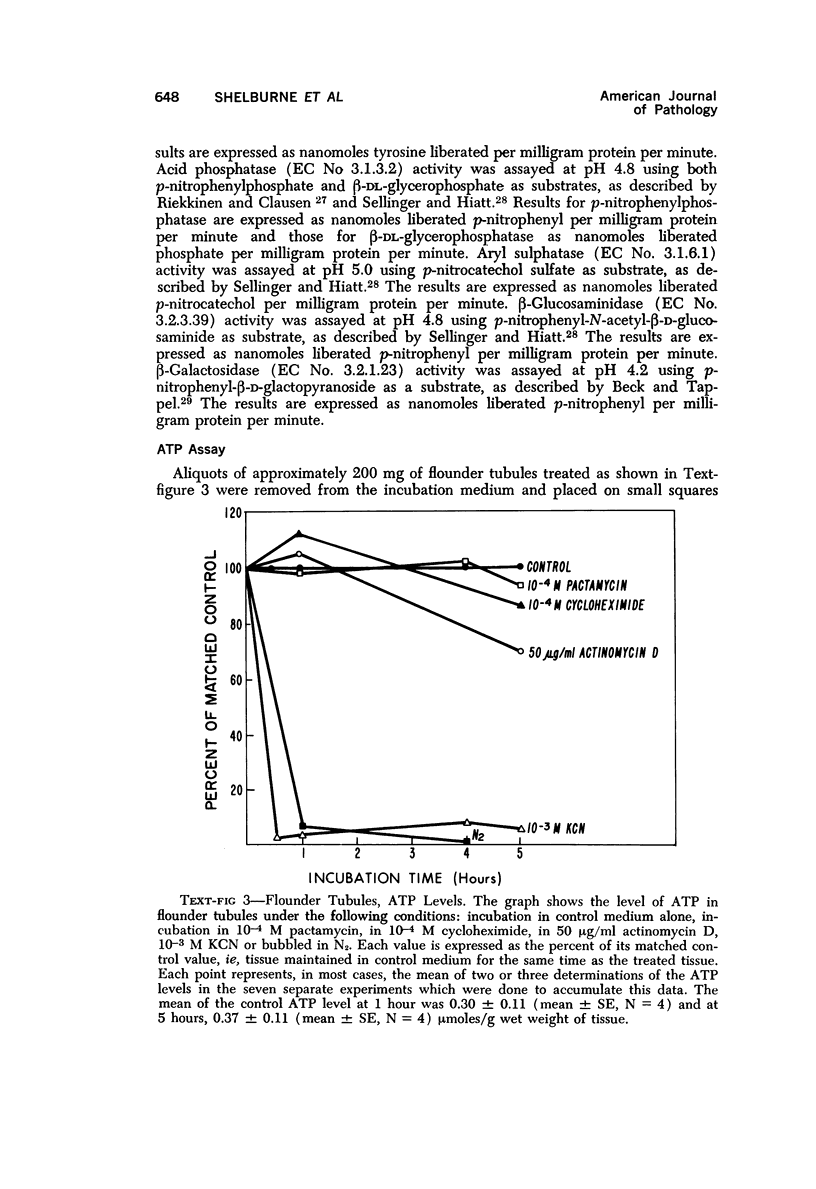
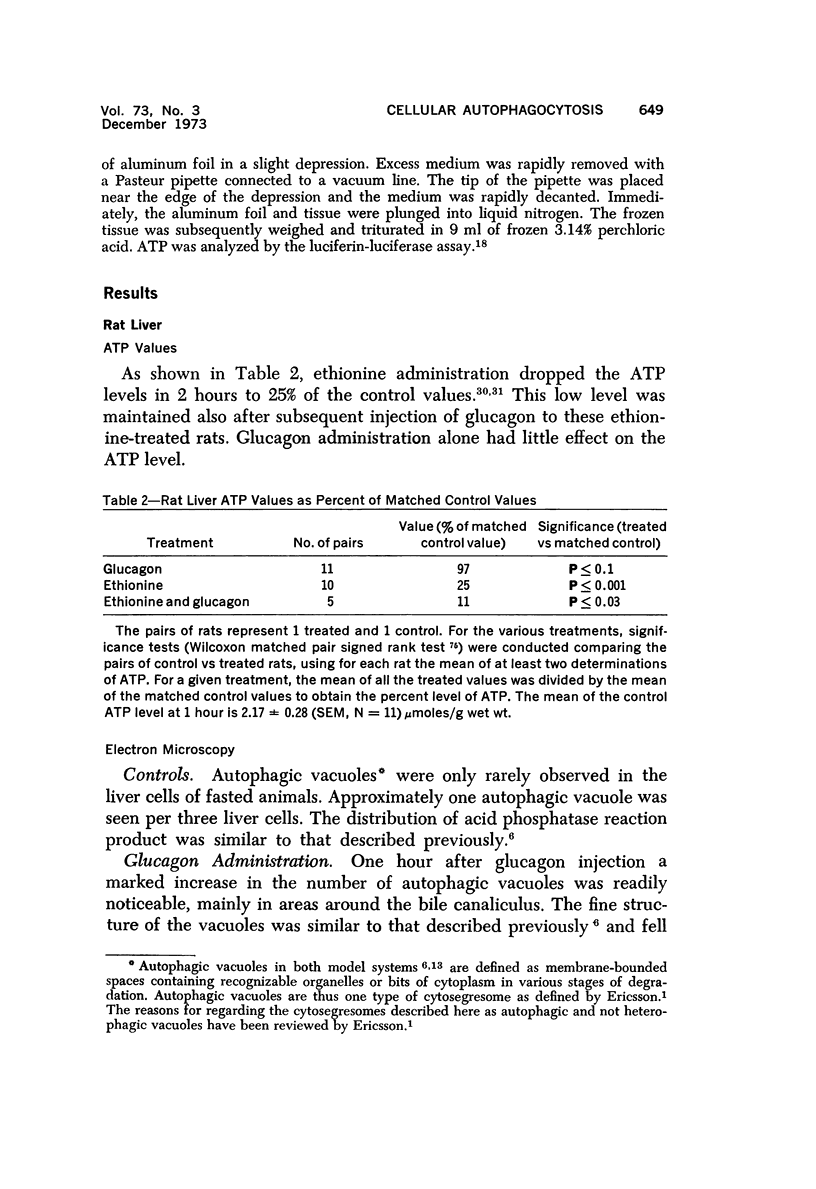
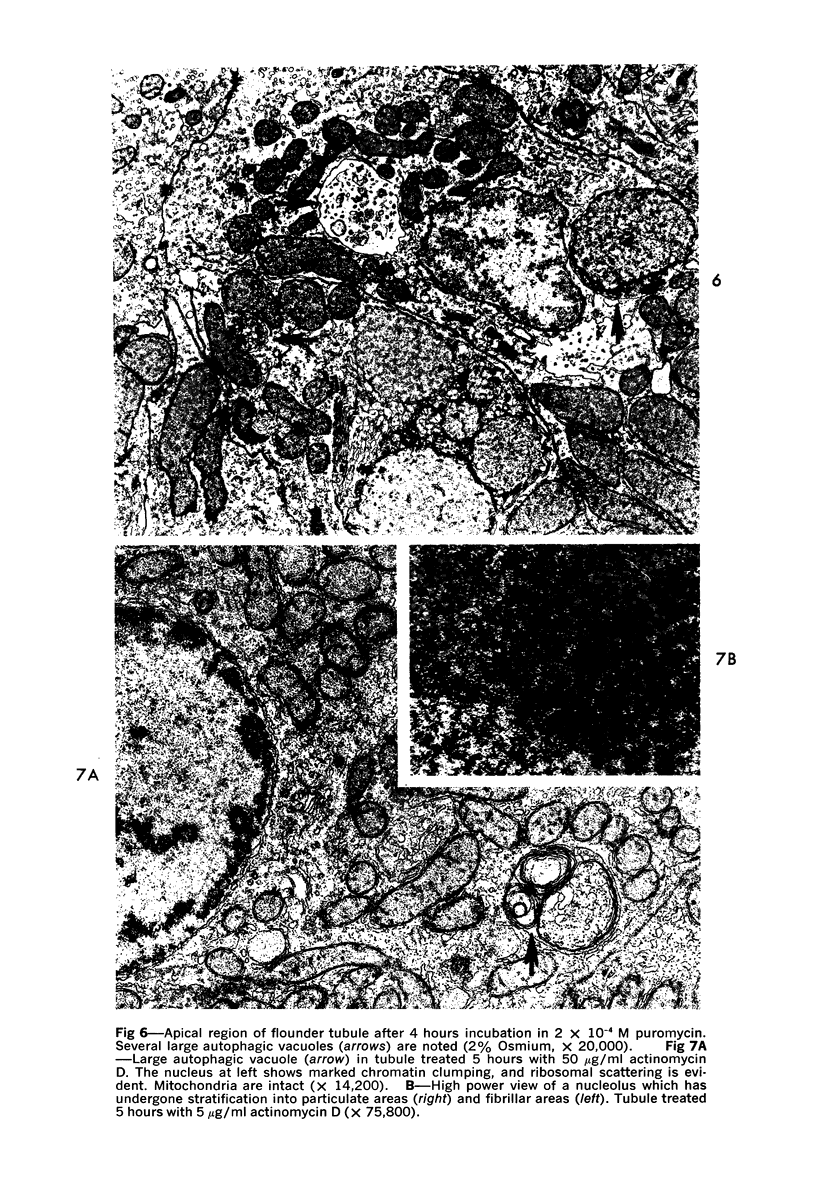
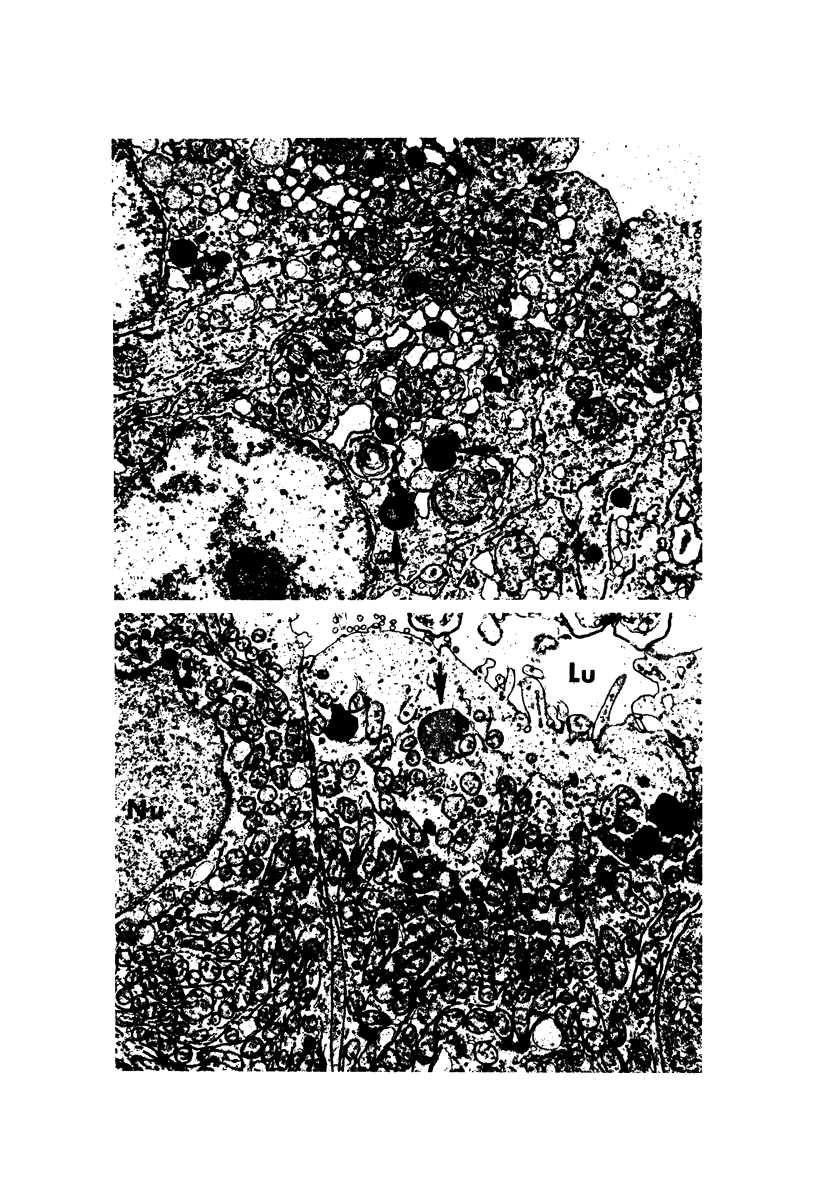
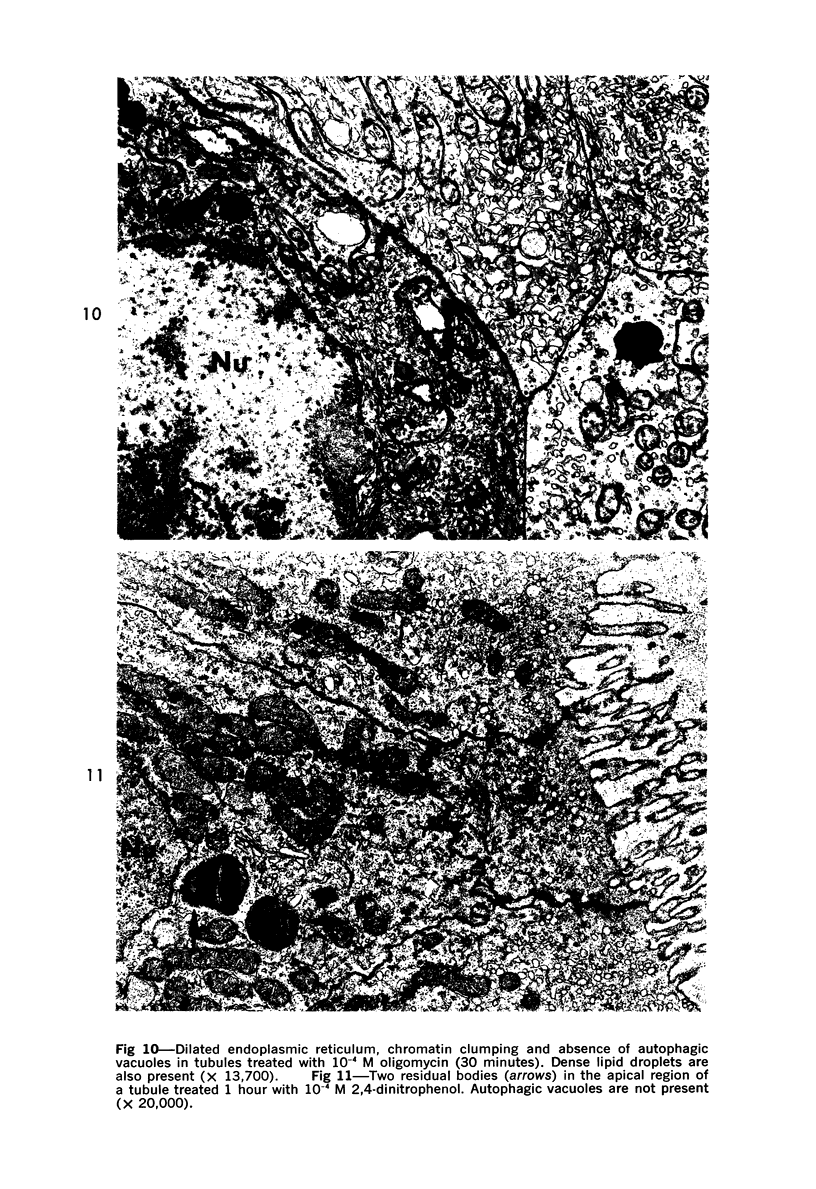
The purpose of the present study was to elucidate the metabolic requirements of autophagocytosis. Two model systems were used for this purpose: a) glucagon-induced autophagocytosis in the rat liver, and b) the wave of autophagocytosis which occurs when isolated flounder kidney tubules are incubated in vitro. In the rat liver, protein synthesis was inhibited by the administration of cycloheximide (1.5 mg/kg) to rats 2 hours prior to glucagon injection. In flounder kidney tubules, protein synthesis was inhibited at least 90% by adding cycloheximide, actinomycin D, pactamycin and puromycin to the medium. In both systems the inhibition of protein synthesis failed to inhibit the formation of autophagic vacuoles or their subsequent transformation into autolysosomes, as depicted from electron microscopic histochemical preparations. In flounder kidney tubules no differences were found in the levels of p-nitrophenylphosphates, β-DL-glycerophosphatase, N-acetyl-β-D-glucosaminidase, arylsulphatase, β-D-galactosidase or acid proteinase when tubules were incubated up to 5 hours in the presence or absence of protein synthesis inhibitors. When ethionine was administered to rats 2 hours before glucagon injection, a decrease of approximately 75% in the ATP levels was observed. After ethionine administration, glucagon failed to induce the formation of autophagic vacuoles. The incubation of flounder kidney tubules in the presence of cyanide or in a nitrogen atmosphere decreased the ATP levels to less than 10% of controls and blocked autophagy. On the other hand, cyanide had little effect on acid hydrolase levels at 1 hour of incubation. A wide variety of other inhibitors were also shown to block autophagy. These results further support the hypothesis that, in the formation of antophagic vacuoles, preexisting enzyme and membrane pools are utilized. On the other hand, the esotropy-exotropy membrane conformational changes occurring in the formation of autophagic vacuoles seem to be energy dependent and can therefore be blocked by lowering intracellular ATP levels.
Full text
PDF
















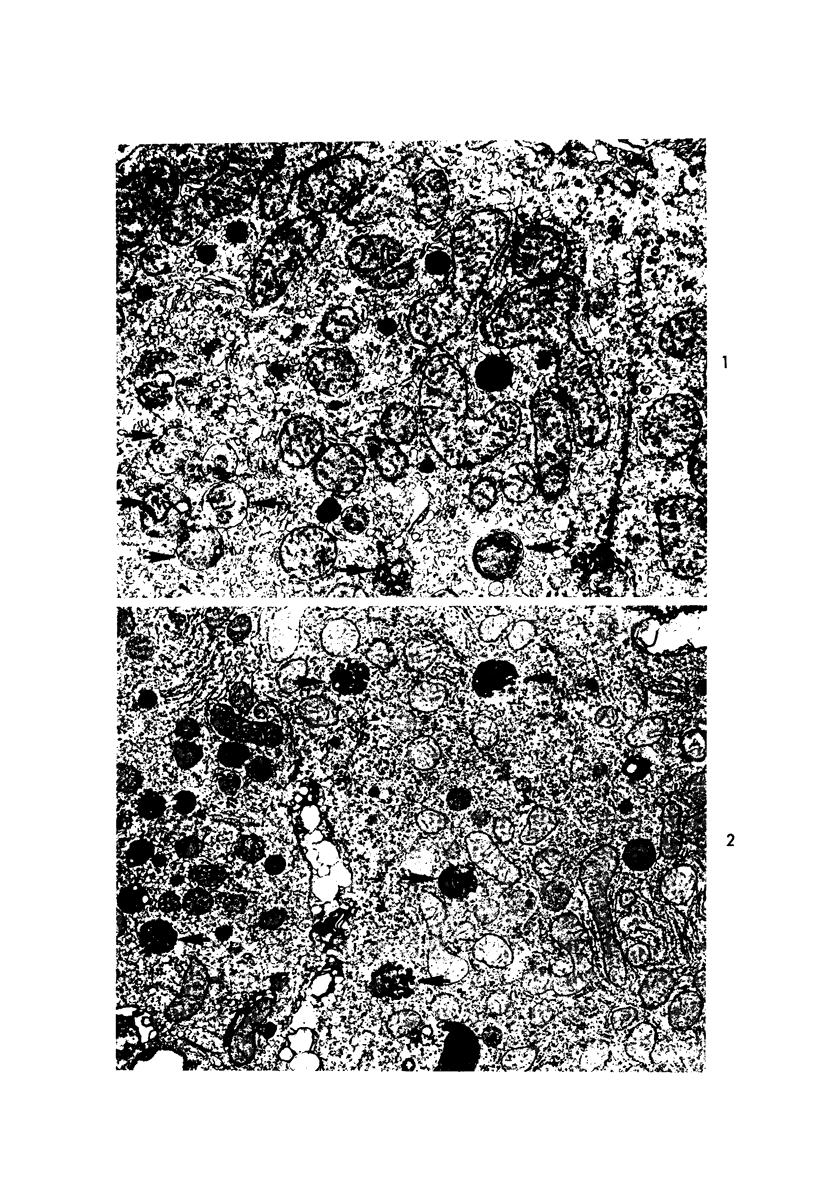
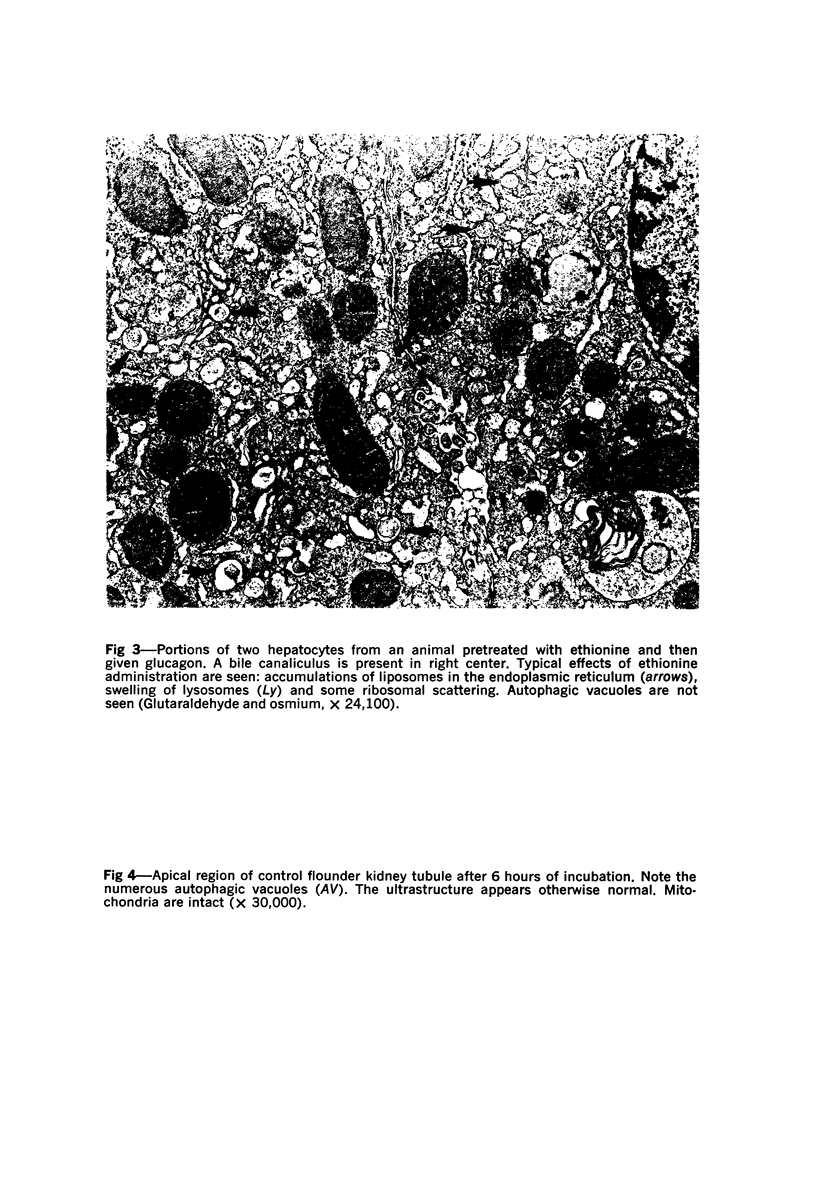
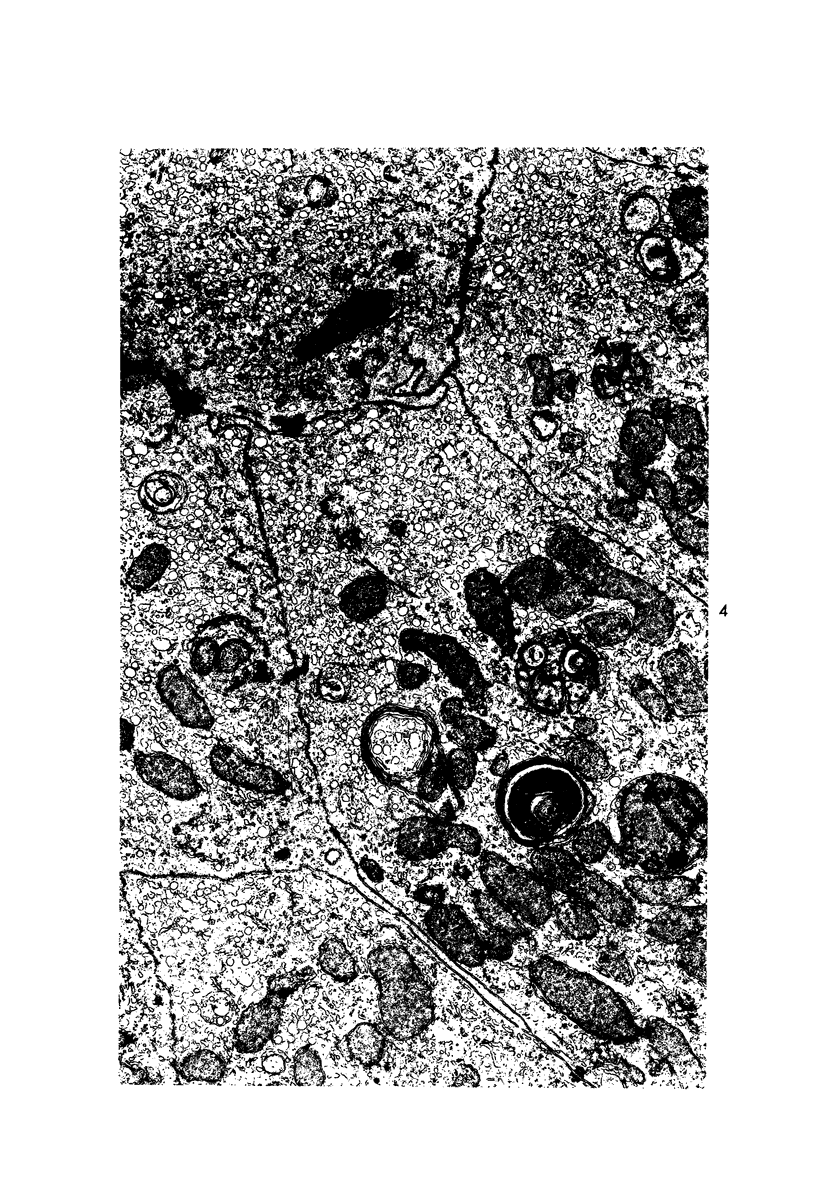
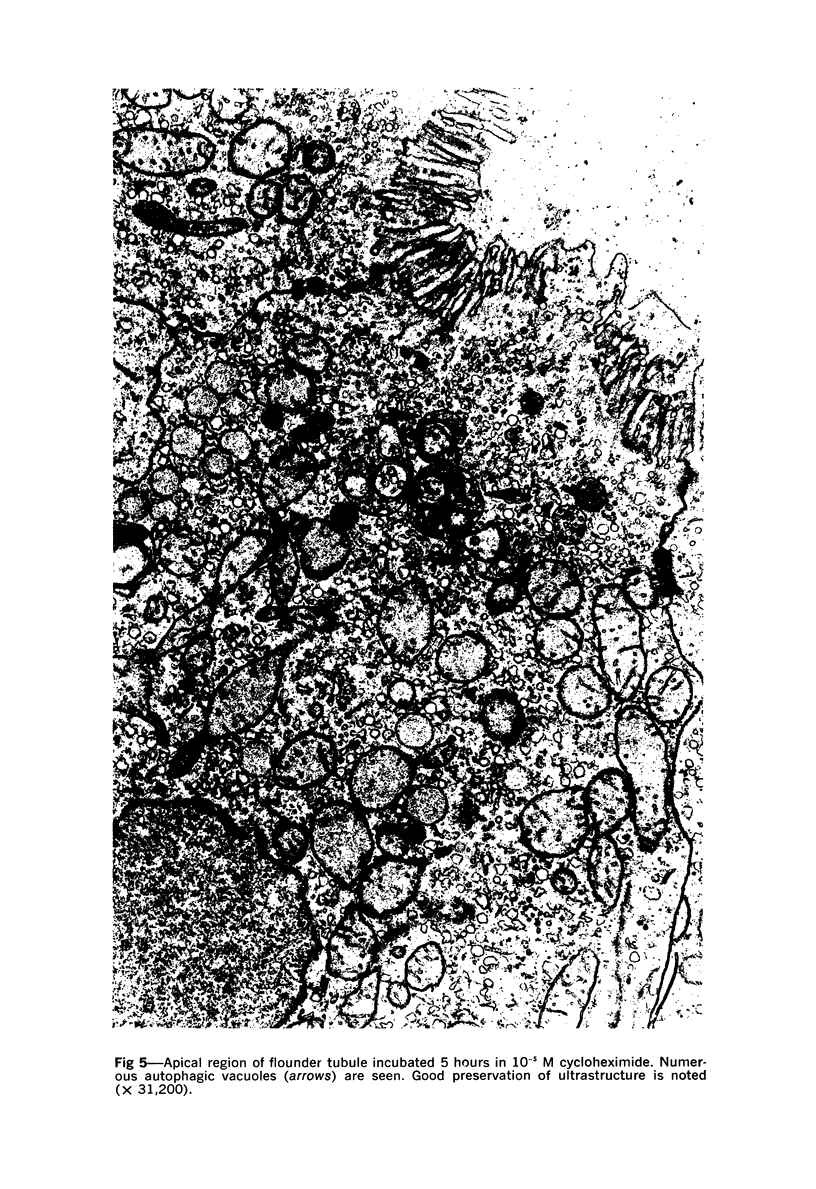

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHFORD T. P., PORTER K. R. Cytoplasmic components in hepatic cell lysosomes. J Cell Biol. 1962 Jan;12:198–202. doi: 10.1083/jcb.12.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arstila A. U., Bradford W. D., Kinney T. D., Trump B. F. Iron metabolism and cell membranes. II. The relationship of ferritin to the cytocavitary network in rat hepatic parenchymal cells. Am J Pathol. 1970 Mar;58(3):419–450. [PMC free article] [PubMed] [Google Scholar]
- Arstila A. U., Jauregui H. O., Chang J., Trump B. F. Studies on cellular autophagocytosis. Relationship between heterophagy and autophagy in HeLa cells. Lab Invest. 1971 Feb;24(2):162–174. [PubMed] [Google Scholar]
- Arstila A. U., Shelburne J. D., Trump B. F. Studies on cellular autophagocytosis. A histochemical study on sequential alterations of mitochondria in the glucagon-induced autophagic vacuoles of rat liver. Lab Invest. 1972 Sep;27(3):317–323. [PubMed] [Google Scholar]
- Arstila A. U., Trump B. F. Ethionine induced alterations in the Golgi apparatus and in the endoplasmic reticulum. Virchows Arch B Cell Pathol. 1972;10(4):344–353. doi: 10.1007/BF02899744. [DOI] [PubMed] [Google Scholar]
- Arstila A. U., Trump B. F. Studies on cellular autophagocytosis. The formation of autophagic vacuoles in the liver after glucagon administration. Am J Pathol. 1968 Nov;53(5):687–733. [PMC free article] [PubMed] [Google Scholar]
- BAGLIO C. M., FARBER E. CORRESPONDENCE BETWEEN RIBOSOME AGGREGATION PATTERNS IN RAT LIVER HOMOGENATES AND IN ELECTRON MICROGRAPHS FOLLOWING ADMINISTRATION OF ETHIONINE. J Mol Biol. 1965 Jun;12:466–467. doi: 10.1016/s0022-2836(65)80269-8. [DOI] [PubMed] [Google Scholar]
- BHUYAN B. K. Pactamycin production by Streptomyces pactum. Appl Microbiol. 1962 Jul;10:302–304. doi: 10.1128/am.10.4.302-304.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliga B. S., Pronczuk A. W., Munro H. N. Mechanism of cycloheximide inhibition of protein synthesis in a cell-free system prepared from rat liver. J Biol Chem. 1969 Aug 25;244(16):4480–4489. [PubMed] [Google Scholar]
- Beck C., Tappel A. L. Rat-liver lysosomal beta-glucosidase: a membrane enzyme. Biochim Biophys Acta. 1968 Jan 8;151(1):159–164. doi: 10.1016/0005-2744(68)90170-8. [DOI] [PubMed] [Google Scholar]
- Bhuyan B. K. Pactamycin, an antibiotic that inhibits protein synthesis. Biochem Pharmacol. 1967 Aug;16(8):1411–1420. doi: 10.1016/0006-2952(67)90116-5. [DOI] [PubMed] [Google Scholar]
- Bread N. S., Jr, Armentrout S. A., Weisberger A. S. Inhibition of mammalian protein synthesis by antibiotics. Pharmacol Rev. 1969 Sep;21(3):213–245. [PubMed] [Google Scholar]
- CARO L. G., PALADE G. E. PROTEIN SYNTHESIS, STORAGE, AND DISCHARGE IN THE PANCREATIC EXOCRINE CELL. AN AUTORADIOGRAPHIC STUDY. J Cell Biol. 1964 Mar;20:473–495. doi: 10.1083/jcb.20.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Goldberg I. H. Inhibition of peptidyl-sRNA binding to ribosomes by pactamycin. Biochem Biophys Res Commun. 1967 Dec 15;29(5):617–622. doi: 10.1016/0006-291x(67)90260-4. [DOI] [PubMed] [Google Scholar]
- Colombo B., Felicetti L., Baglioni C. Inhibition of protein synthesis in reticulocytes by antibiotics. I. Effects on polysomes. Biochim Biophys Acta. 1966 Apr 18;119(1):109–119. doi: 10.1016/0005-2787(66)90043-8. [DOI] [PubMed] [Google Scholar]
- Deter R. L., Baudhuin P., De Duve C. Participation of lysosomes in cellular autophagy induced in rat liver by glucagon. J Cell Biol. 1967 Nov;35(2):C11–C16. doi: 10.1083/jcb.35.2.c11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deter R. L., De Duve C. Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes. J Cell Biol. 1967 May;33(2):437–449. doi: 10.1083/jcb.33.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deter R. L. Quantitative characterization of dense body, autophagic vacuole, and acid phosphatase-bearing particle populations during the early phases of glucagon-induced autophagy in rat liver. J Cell Biol. 1971 Mar;48(3):473–489. doi: 10.1083/jcb.48.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson J. L. Studies on induced cellular autophagy. II. Characterization of the membranes bordering autophagosomes in parenchymal liver cells. Exp Cell Res. 1969 Aug;56(2):393–405. doi: 10.1016/0014-4827(69)90030-5. [DOI] [PubMed] [Google Scholar]
- Ericsson J. L., Trump B. F. Observations on the application to electron microscopy on the lead phosphate technique for the demonstration of acid phosphatase. Histochemie. 1965 Mar 5;4(6):470–487. doi: 10.1007/BF00281900. [DOI] [PubMed] [Google Scholar]
- Farber E. Biochemical pathology. Annu Rev Pharmacol. 1971;11:71–96. doi: 10.1146/annurev.pa.11.040171.000443. [DOI] [PubMed] [Google Scholar]
- Felicetti L., Colombo B., Baglioni C. Inhibition of protein synthesis in reticulocytes by antibiotics. II. The site of action of cycloheximide, streptovitacin A and pactamycin. Biochim Biophys Acta. 1966 Apr 18;119(1):120–129. [PubMed] [Google Scholar]
- Forster R. P. Use of Thin Kidney Slices and Isolated Renal Tubules for Direct Study of Cellular Transport Kinetics. Science. 1948 Jul 16;108(2794):65–67. doi: 10.1126/science.108.2794.65-a. [DOI] [PubMed] [Google Scholar]
- Ginn F. L., Shelburne J. D., Trump B. F. Disorders of cell volume regulation. I. Effects of inhibition of plasma membrane adenosine triphosphatase with ouabain. Am J Pathol. 1968 Dec;53(6):1041–1071. [PMC free article] [PubMed] [Google Scholar]
- Goldblatt P. J., Sullivan R. J., Farber E. Morphologic and metabolic alterations in hepatic cell nucleoli induced by varying doses of actinomycin D. Cancer Res. 1969 Jan;29(1):124–135. [PubMed] [Google Scholar]
- Goldblatt P. J., Williams G. M. Some alterations in hepatic cytosome structure and acid phosphatase activity induced by DL-ethionine. Am J Pathol. 1969 Nov;57(2):253–271. [PMC free article] [PubMed] [Google Scholar]
- Goldblatt P. J., Witschi H., Friedman M. A., Sullivan R. J., Shull K. H. Some structural and functional consequences of hepatic adenosine triphosphate deficiency induced by intraperitoneal D-fructose administration. Lab Invest. 1970 Oct;23(4):378–385. [PubMed] [Google Scholar]
- Knodell R. G., Toskes P. P., Reber H. A., Brooks F. P. Significance of cyclic AMP in the regulation of exocrine pancreas secretion. Experientia. 1970 May 15;26(5):515–517. doi: 10.1007/BF01898480. [DOI] [PubMed] [Google Scholar]
- Kumon A., Yamamura H., Nishizuka Y. Mode of action of adenosine 3',5'-cyclic phosphate on protein kinase from rat liver. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1290–1297. doi: 10.1016/0006-291x(70)90228-7. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laiho K. U., Shelburne J. D., Trump B. F. Observations on cell volume, ultrastructure, mitochondrial conformation and vital-dye uptake in Ehrlich ascites tumor cells. Effects of inhibiting energy production and function of the plasma membrane. Am J Pathol. 1971 Oct;65(1):203–230. [PMC free article] [PubMed] [Google Scholar]
- Lardy H. A., Ferguson S. M. Oxidative phosphorylation in mitochondria. Annu Rev Biochem. 1969;38:991–1034. doi: 10.1146/annurev.bi.38.070169.005015. [DOI] [PubMed] [Google Scholar]
- Laszlo J., Miller D. S., McCarty K. S., Hochstein P. Actinomycin D: inhibition of respiration and glycolysis. Science. 1966 Feb 25;151(3713):1007–1010. doi: 10.1126/science.151.3713.1007. [DOI] [PubMed] [Google Scholar]
- Lin S. Y., Mosteller R. D., Hardesty B. The mechanism of sodium fluoride and cycloheximide inhibition of hemoglobin biosynthesis in the cell-free reticulocyte system. J Mol Biol. 1966 Oct 28;21(1):51–69. doi: 10.1016/0022-2836(66)90079-9. [DOI] [PubMed] [Google Scholar]
- Penniston J. T., Green D. E. The conformational basis of energy transformations in membrane systems. IV. Energized states and pinocytosis in erythrocyte ghosts. Arch Biochem Biophys. 1968 Nov;128(2):339–350. doi: 10.1016/0003-9861(68)90040-4. [DOI] [PubMed] [Google Scholar]
- Prose P. H., Balk S. D., Liebhaber H., Krugman S. Studies of a myxovirus recovered from patients with infectious hepatitis. II. Fine structure and electron microscopic demonstration of intracytoplasmic internal component and viral filament formation. J Exp Med. 1965 Dec 1;122(6):1151–1160. doi: 10.1084/jem.122.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Riekkinen P. J., Clausen J. Proteinase activity of myelin. Brain Res. 1969 Oct;15(2):413–430. doi: 10.1016/0006-8993(69)90164-4. [DOI] [PubMed] [Google Scholar]
- SHULL K. H. Hepatic phosphorylase and adenosine triphosphate levels in ethionine-treated rats. J Biol Chem. 1962 May;237:1734–1735. [PubMed] [Google Scholar]
- Sahaphong S., Trump B. F. Studies of cellular injury in isolated kidney tubules of the flounder. V. Effects of inhibiting sulfhydryl groups of plasma membrane with the organic mercurials PCMB (parachloromercuribenzoate) and PCMB (parachloromercuribenzenesulfonate). Am J Pathol. 1971 May;63(2):277–298. [PMC free article] [PubMed] [Google Scholar]
- Sellinger O. Z., Hiatt R. A. Cerebral lysosomes. IV. The regional and intracellular distribution of arylsulfatase and evidence for two populations of lysosomes in rat brain. Brain Res. 1968 Feb;7(2):191–200. doi: 10.1016/0006-8993(68)90097-8. [DOI] [PubMed] [Google Scholar]
- Shelburne J. D., Arstila A. U., Trump B. F. Studies on cellular autophagocytosis. Cyclic AMP- and dibutyryl cyclic AMP-stimulated autophagy in rat liver. Am J Pathol. 1973 Sep;72(3):521–540. [PMC free article] [PubMed] [Google Scholar]
- Smuckler E. A., Benditt E. P. The early effects of actinomycin on rat liver. Changes in ribosomes and polysomes. Lab Invest. 1965 Oct;14(10):1699–1709. [PubMed] [Google Scholar]
- Trump B. F., Bulger R. E. Studies of cellular injury in isolated flounder tubules. I. Correlation between morphology and function of control tubules and observations of autophagocytosis and mechanical cell damage. Lab Invest. 1967 Mar;16(3):453–482. [PubMed] [Google Scholar]
- Trump B. F., Bulger R. E. Studies of cellular injury in isolated flounder tubules. IV. Electron microscopic observations of changes during the phase of altered homeostasis in tubules treated with cyanide. Lab Invest. 1968 Jun;18(6):731–739. [PubMed] [Google Scholar]
- VILLA-TREVINO S., SHULL K. H., FARBER E. The role of adenosine triphosphate deficiency in ethionine-induced inhibition of protein synthesis. J Biol Chem. 1963 May;238:1757–1763. [PubMed] [Google Scholar]
- Verbin R. S., Goldblatt P. J., Farber E. The biochemical pathology of inhibition of protein synthesis in vivo. The effects of cycloheximide on hepatic parenchymal cell ultrastructure. Lab Invest. 1969 Jun;20(6):529–536. [PubMed] [Google Scholar]
- Verbin R. S., Sullivan R. J., Farber E. The effects of cycloheximide on the cell cycle of the regenerating rat liver. Lab Invest. 1969 Aug;21(2):179–182. [PubMed] [Google Scholar]
- WHEELOCK E. F. The role of protein synthesis in the eclipse period of newcastle disease virus multiplication in HeLa cells as studied with puromycin. Proc Natl Acad Sci U S A. 1962 Aug;48:1358–1366. doi: 10.1073/pnas.48.8.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh S. D., Shils M. E. Quantitive aspects of cycloheximide inhibition of amino acid incorporation. Biochem Pharmacol. 1969 Aug;18(8):1919–1926. doi: 10.1016/0006-2952(69)90287-1. [DOI] [PubMed] [Google Scholar]