Abstract
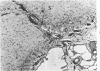
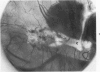
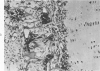
If puppy tissues are explanted onto the chick chorioallantoic membrane, those tissues which normally have a blood supply are rapidly invaded by vascularized mesenchyme of host origin. Hyaline cartilage, a tissue virtually devoid of blood vessels, is impenetrable by proliferating mesenchyme of the host, while calcified cartilage, which normally is vascularized, is penetrable. The stroma of the cornea, another normally avascular tissue, is readily penetrable, but Descemet's membrane forms a barrier to invasion by host tissues. The experimental system used permits the design of experiments in which the study of factors responsible for the resistance of tissues such as cartilage to invasion can be undertaken.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Folkman J., Merler E., Abernathy C., Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med. 1971 Feb 1;133(2):275–288. doi: 10.1084/jem.133.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971 Nov 18;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Leapman S. B., Cotran R. S., Folkman J. Tumor angiogenesis: iris neovascularization at a distance from experimental intraocular tumors. J Natl Cancer Inst. 1973 Jan;50(1):219–228. doi: 10.1093/jnci/50.1.219. [DOI] [PubMed] [Google Scholar]
- Hirano A., Zimmerman H. M. Fenestrated blood vessels in a metastatic renal carcinoma in the brain. Lab Invest. 1972 Apr;26(4):465–468. [PubMed] [Google Scholar]
- Irving J. T., Heeley J. D. The effect of enzyme treatment upon the resorption of scapular implants. Calcif Tissue Res. 1970;5(1):64–69. doi: 10.1007/BF02017535. [DOI] [PubMed] [Google Scholar]
- Kuettner K. E., Soble L. W., Guenther H. L., Croxen R. L., Eisenstein R. Lysozyme in epiphyseal cartilage. I. The nature of the morphologic response of cartilage in culture to exogenous lysozym. Calcif Tissue Res. 1970;5(1):56–63. doi: 10.1007/BF02017534. [DOI] [PubMed] [Google Scholar]
- McKibbin B., Holdsworth F. W. The dual nature of epiphysial cartilage. J Bone Joint Surg Br. 1967 May;49(2):351–361. [PubMed] [Google Scholar]
- SABET T. Y., HIDVEGI E. B., RAY R. D. Bone immunology. II. Comparison of embryonic mouse isografts and homografts. J Bone Joint Surg Am. 1961 Oct;43-A:1007–1021. [PubMed] [Google Scholar]
- Sajdera S. W., Hascall V. C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969 Jan 10;244(1):77–87. [PubMed] [Google Scholar]
- Slavkin H. C., Bringas P., Cameron J., LeBaron R., Bavetta L. A. Epithelial and mesenchymal cell interactions with extracellular matrix material in vitro. J Embryol Exp Morphol. 1969 Nov;22(3):395–405. [PubMed] [Google Scholar]
- Wilsman N. J., Van Sickle D. C. Cartilage canals, their morphology and distribution. Anat Rec. 1972 May;173(1):79–93. doi: 10.1002/ar.1091730107. [DOI] [PubMed] [Google Scholar]