Abstract
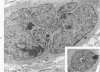
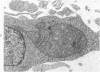
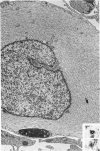
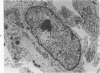
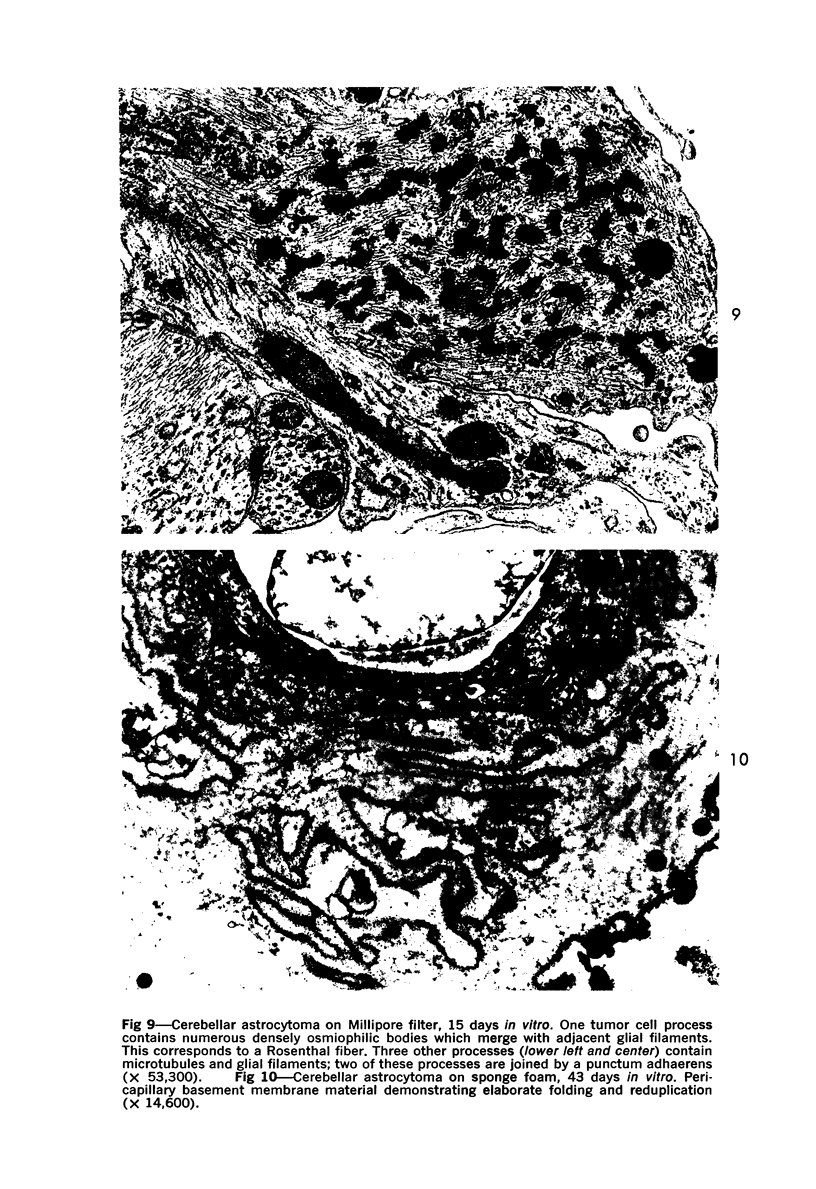
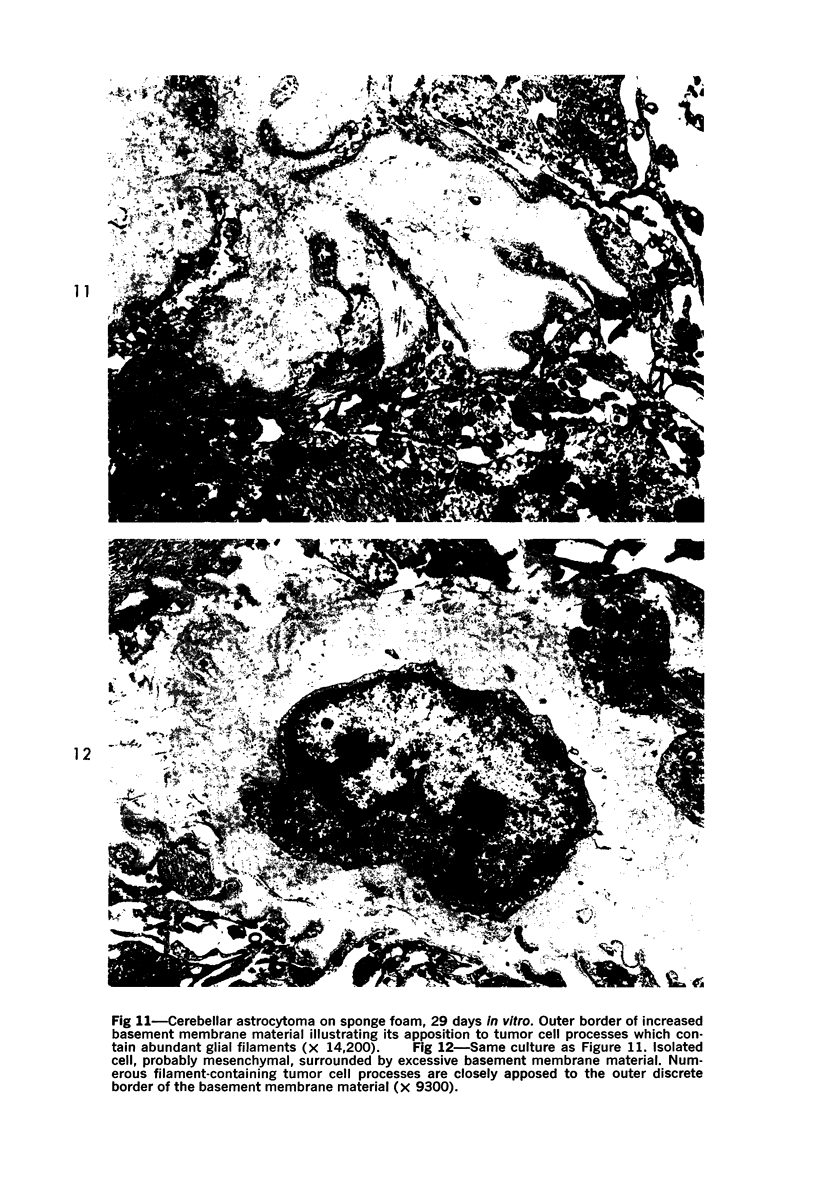
The fine structure of four glioblastomas and two cerebellar astrocytomas maintained in organ culture systems up to 137 days and 43 days, respectively, using either a three-dimensional sponge foam matrix technic or a Millipore filter platform technic, is described and compared. The cells of both tumor types showed increased astrocytic differentiation, characterized by a progressive increase in glial filaments associated with an increase in free ribosomes and granular endoplasmic reticulum. A progressive increase in basement membrane material, presumably originating from explanted endothelial cells or pericytes, was also found in both tumor types and was often associated with increased numbers of collagen fibrils. Astrocytic tumor cell processes frequently preserved their contact with this basement membrane material. Microvascular fenestrations or gaps in endothelial cells were not identified. These electron microscopic features appear to correspond to the early stages of perivascular sclerosis previously noted by light microscopy in gliomas maintained in organ culture systems and are presumably related to the progressive obliteration of the functional microvasculature.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUNGE R. P., BUNGE M. B., PETERSON E. R. AN ELECTRON MICROSCOPE STUDY OF CULTURED RAT SPINAL CORD. J Cell Biol. 1965 Feb;24:163–191. doi: 10.1083/jcb.24.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami A., Eng L. F., Dahl D., Uyeda C. T. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972 Aug 25;43(2):429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Buckley I. K., Porter K. R. Cytoplasmic fibrils in living cultured cells. A light and electron microscope study. Protoplasma. 1967;64(4):349–380. doi: 10.1007/BF01666538. [DOI] [PubMed] [Google Scholar]
- Caley D. W., Maxwell D. S. Development of the blood vessels and extracellular spaces during postnatal maturation of rat cerebral cortex. J Comp Neurol. 1970 Jan;138(1):31–47. doi: 10.1002/cne.901380104. [DOI] [PubMed] [Google Scholar]
- Cravioto H., Lockwood R. Long-spacing fibrous collagen in human acoustic nerve tumors. In vvo and in vitro observations. J Ultrastruct Res. 1968 Jul;24(1):70–85. doi: 10.1016/s0022-5320(68)80017-6. [DOI] [PubMed] [Google Scholar]
- DUFFELL D., FARBER L., CHOU S., HARTMANN J. F., NELSON E. ELECTRON MICROSCOPIC OBSERVATIONS ON ASTROCYTOMAS. Am J Pathol. 1963 Oct;43:539–545. [PMC free article] [PubMed] [Google Scholar]
- Eng L. F., Vanderhaeghen J. J., Bignami A., Gerstl B. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971 May 7;28(2):351–354. doi: 10.1016/0006-8993(71)90668-8. [DOI] [PubMed] [Google Scholar]
- Gullotta F., Fliedner E. Spongioblastomas, astrocytomas and Rosenthal fibers. Ultrastructural, tissue culture and enzyme histochemical investigations. Acta Neuropathol. 1972;22(1):68–78. doi: 10.1007/BF00687551. [DOI] [PubMed] [Google Scholar]
- Hirano A., Dembitzer H. M., Zimmerman H. M. Fenestrated blood vessels in neurilemoma. Lab Invest. 1972 Sep;27(3):305–309. [PubMed] [Google Scholar]
- Holmström T., Saksela E., Nyström S., Saxén E. Growth behaviour of human brain tumours in matrix cultures in fresh autologous serum. Acta Pathol Microbiol Scand A. 1970;78(3):313–322. doi: 10.1111/j.1699-0463.1970.tb03307.x. [DOI] [PubMed] [Google Scholar]
- Hossmann K. A., Wechsler W. Zur Feinstruktur menschlicher Spongioblastome. Dtsch Z Nervenheilkd. 1965 Jul 21;187(4):327–351. [PubMed] [Google Scholar]
- Karnovsky M. J. The ultrastructural basis of capillary permeability studied with peroxidase as a tracer. J Cell Biol. 1967 Oct;35(1):213–236. doi: 10.1083/jcb.35.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUSE S. A. Electron microscopic studies of brain tumors. Neurology. 1960 Oct;10:881–905. doi: 10.1212/wnl.10.10.881. [DOI] [PubMed] [Google Scholar]
- Long D. M. Capillary ultrastructure and the blood-brain barrier in human malignant brain tumors. J Neurosurg. 1970 Feb;32(2):127–144. doi: 10.3171/jns.1970.32.2.0127. [DOI] [PubMed] [Google Scholar]
- Lyser K. M. The differentiation of glial cells and glia limitans in organ cultures of chick spinal cord. In Vitro. 1972 Sep-Oct;8(2):77–84. doi: 10.1007/BF02615963. [DOI] [PubMed] [Google Scholar]
- Pontén J., Macintyre E. H. Long term culture of normal and neoplastic human glia. Acta Pathol Microbiol Scand. 1968;74(4):465–486. doi: 10.1111/j.1699-0463.1968.tb03502.x. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON K. C., JARETT L., FINKE E. H. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 1960 Nov;35:313–323. doi: 10.3109/10520296009114754. [DOI] [PubMed] [Google Scholar]
- Rawlinson D. G., Herman M. M., Rubinstein L. J. The fine structure of a myxopapillary ependymoma of the filum terminale. Acta Neuropathol. 1973 Jun 26;25(1):1–13. doi: 10.1007/BF00686853. [DOI] [PubMed] [Google Scholar]
- Rubinstein L. J., Herman M. M. A light- and electron-microscopic study of a temporal-lobe ganglioglioma. J Neurol Sci. 1972 May;16(1):27–48. doi: 10.1016/0022-510x(72)90100-1. [DOI] [PubMed] [Google Scholar]
- Rubinstein L. J., Herman M. M., Foley V. L. In vitro characteristics of human glioblastomas maintained in organ culture systems. Light microscopy observations. Am J Pathol. 1973 Apr;71(1):61–80. [PMC free article] [PubMed] [Google Scholar]
- Rubinstein L. J., Herman M. M., Miquel J., Weibel J. The short- and long-term effects of ultraviolet irradiation on the exposed cat cerebrum. Light-microscopic, enzyme-histochemical and fine-structural observations. J Neurol Sci. 1971 Jul;13(3):351–375. doi: 10.1016/0022-510x(71)90038-4. [DOI] [PubMed] [Google Scholar]
- Schlote W. Rosenthalsche "Fasern" und Spongioblasten im Zentralnervensystem. II. Elektronenmikroskopische Untersuchungen. Bedeutung der Rosenthalschen "Fasern". Beitr Pathol Anat. 1966 Jun;133(4):460–480. [PubMed] [Google Scholar]
- Shein H. M. Propagation of human fetal spongioblasts and astrocytes in dispersed cell cultures. Exp Cell Res. 1965 Dec;40(3):554–569. doi: 10.1016/0014-4827(65)90234-x. [DOI] [PubMed] [Google Scholar]
- Vaughn J. E., Pease D. C. Electron microscopic studies of wallerian degeneration in rat optic nerves. II. Astrocytes, oligodendrocytes and adventitial cells. J Comp Neurol. 1970 Oct;140(2):207–226. doi: 10.1002/cne.901400205. [DOI] [PubMed] [Google Scholar]
- Vick N. A., Bigner D. D. Microvascular abnormalities in virally-induced canine brain tumors. Structural bases for altered blood-brain barrier function. J Neurol Sci. 1972 Sep;17(1):29–39. doi: 10.1016/0022-510x(72)90019-6. [DOI] [PubMed] [Google Scholar]
- Vick N. A., Bigner D. D. Some structural aspects of dog brain tumors induced with the Schmidt-Ruppin strain of the Rous sarcoma virus. Prog Exp Tumor Res. 1972;17:59–73. doi: 10.1159/000393668. [DOI] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M. K., Dubois-Dalcq M. Anatomy of cultured mouse cerebellum. I. Golgi and electron microscopic demonstrations of granule cells, their afferent and efferent synapses. J Comp Neurol. 1970 Nov;140(3):261–280. doi: 10.1002/cne.901400303. [DOI] [PubMed] [Google Scholar]