Abstract
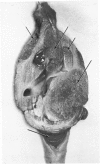
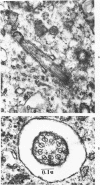
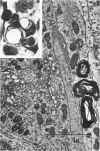
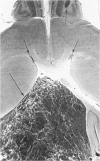
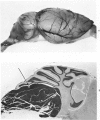
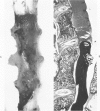
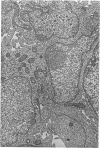
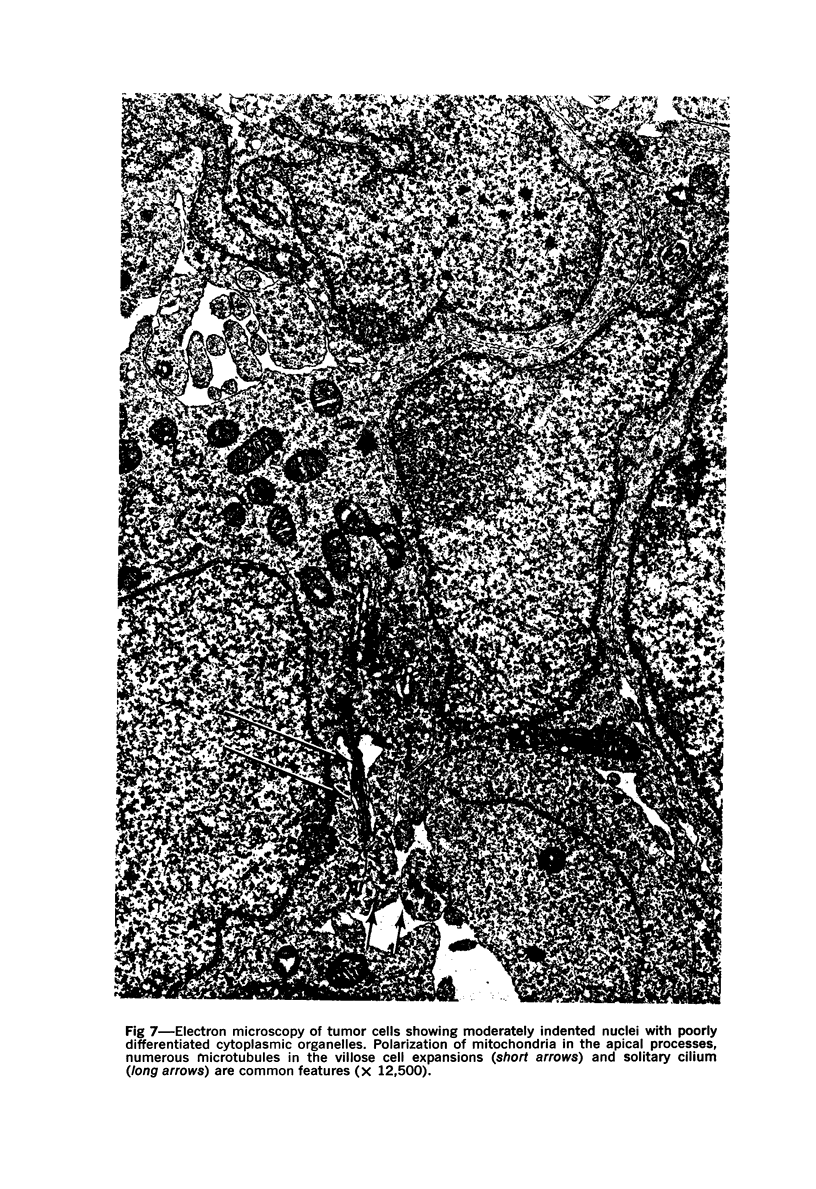
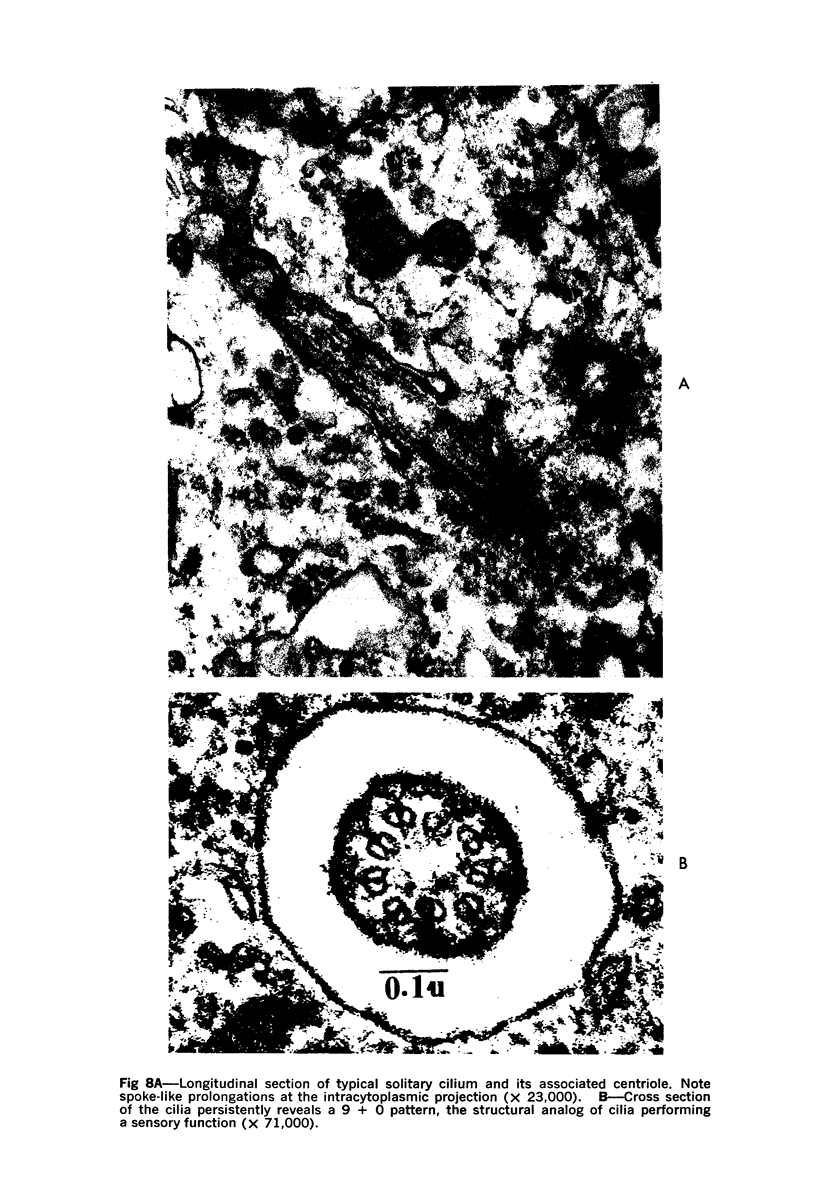
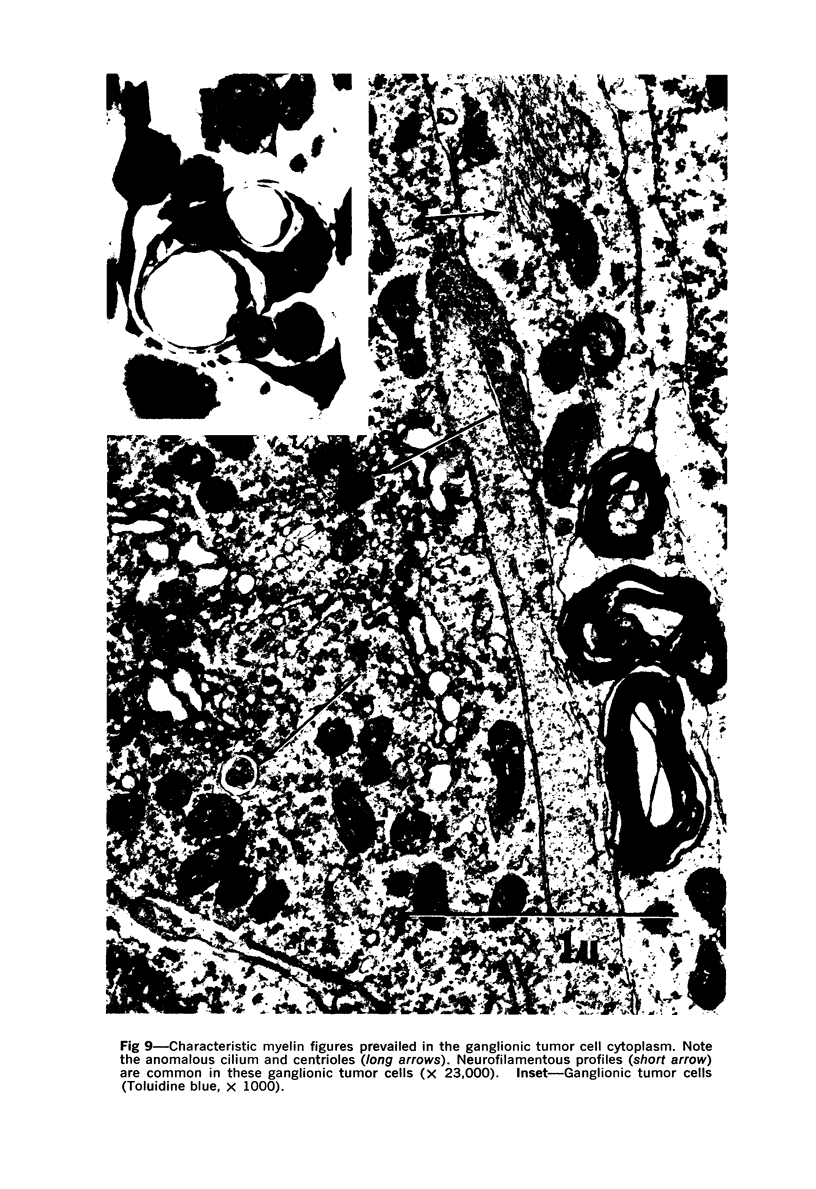
A direct causal relationship between a human virus and malignant transformations in target cells (sensory neuronal precursors) was suggested by the development of a medulloepitheliomatous neoplasm in the central nervous system. Twenty-six newborn Sprague-Dawley rats were given a single intracerebral inoculation of 0.05 ml of adenovirus fluid, 103.5 to 104.5 TCID50 HeLa cells/0.1 ml, in the left frontal lobe. Within 37 to 151 days after the virus inoculation, 23 (88.7%) rats autochthonously developed an adenovirus-typical neoplasm in the central nervous system. Nine animals developed a multicentric neoplasm closely related to the ventricular system. Nine others developed solid variously sized neoplasms along the ventricular lumen. Some neoplasms showed multiple foci connected with the stratum subependymale ventriculi olfactorii and the velum medullare of the fourth ventricle. Six spinal cord tumors, located chiefly in the dorsal sensory column, developed within 37 to 61 days after intracerebral inoculation. The remarkably uniform histopathologic appearance of all 23 cases was attributed to a medulloepitheliomatous neoplasm derived from the ependymal anlage. Electron microscopy clearly revealed a solitary cilium within the apical region of many tumor cells. It consisted of a typical ring of nine doublets with no axial pair (a 9+0 pattern), the typical structure of cilia of sensory neuronal origin. The appearance of exuberant neuron-like tumor cells with argyrophile cytoplasmic expansions, neurosyncytial mosaic alignment and myelin-like configurations also suggested a neuronal origin. A paucity of mesenchymal stroma in the neoplastic tissue was noted. No control animals developed tumors.
Full text
PDF



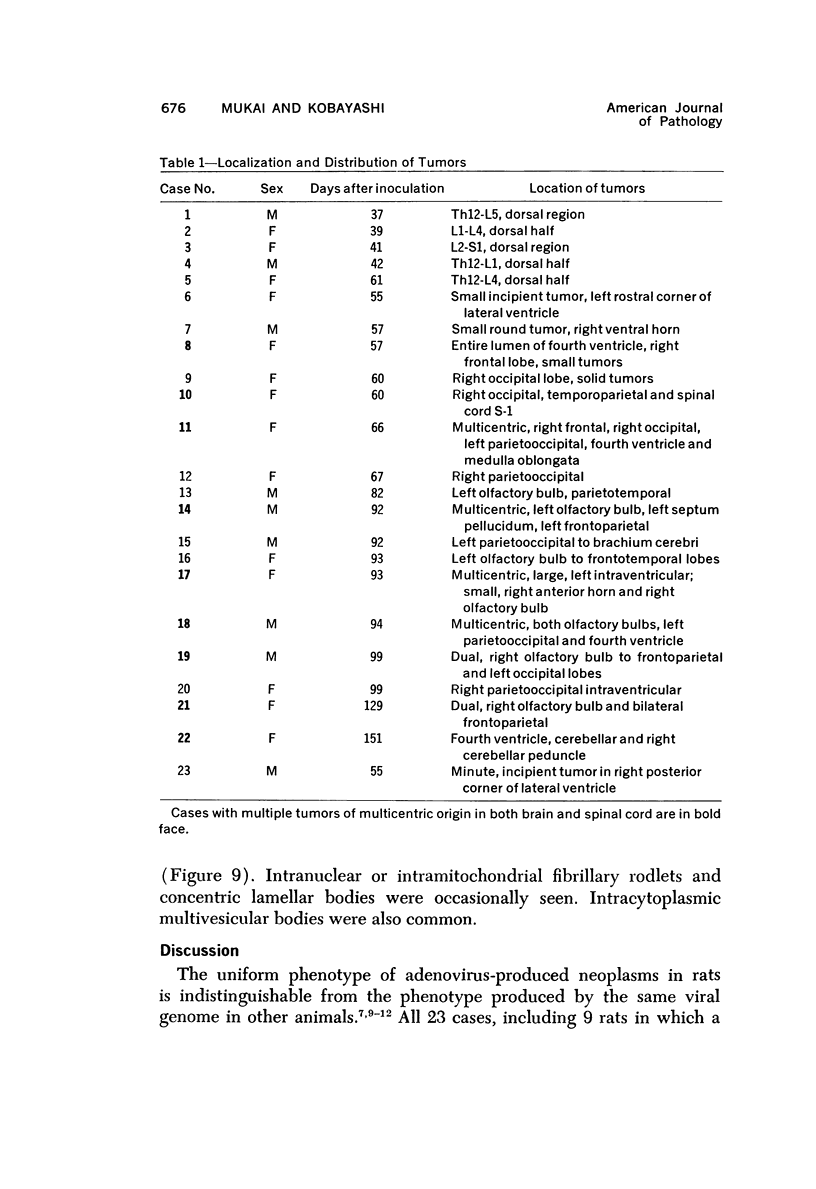





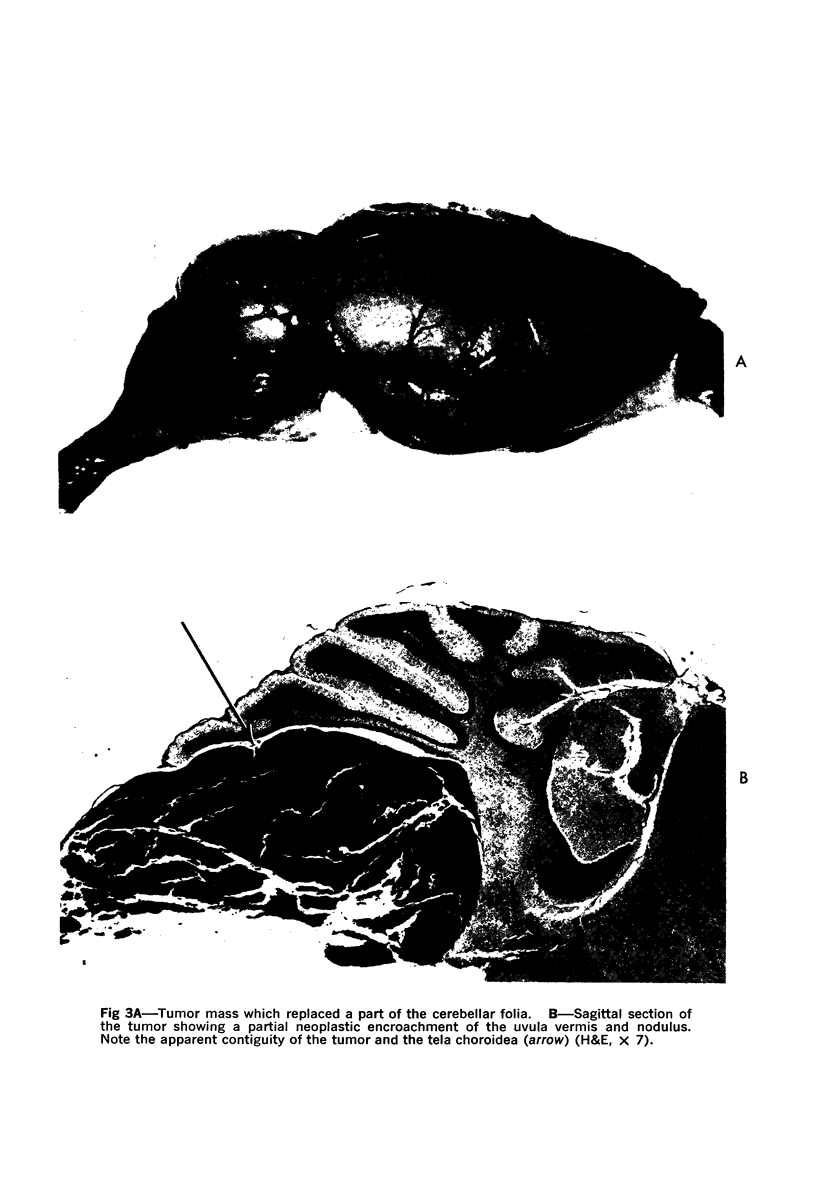



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN R. A., LATTA H., STRAATSMA B. R. Retinoblastoma. A study of two cases by electron microscopy. Invest Ophthalmol. 1962 Dec;1:728–744. [PubMed] [Google Scholar]
- Cervós-Navarro J., Vázquez J. Elektronenmikroskopische Untersuchungen über das Vorkommen von Cilien in Meningeomen. Virchows Arch Pathol Anat Physiol Klin Med. 1966 Dec 14;341(4):280–290. [PubMed] [Google Scholar]
- DAHL H. A. Fine structure of cilia in rat cerebral cortex. Z Zellforsch Mikrosk Anat. 1963;60:369–386. doi: 10.1007/BF00336612. [DOI] [PubMed] [Google Scholar]
- Deck J. H. Cerebral medulloepithelioma with maturation into ependymal cells and ganglion cells. J Neuropathol Exp Neurol. 1969 Jul;28(3):442–454. doi: 10.1097/00005072-196907000-00006. [DOI] [PubMed] [Google Scholar]
- Jellinger K. Cerebral medulloepithelioma. Acta Neuropathol. 1972;22(2):95–101. doi: 10.1007/BF00688777. [DOI] [PubMed] [Google Scholar]
- KIBRICK S., ENDERS J. F., ROBBINS F. C. An evaluation of the roller-tube tissue culture for the isolation of poliomyelitis viruses from feces. J Immunol. 1955 Nov;75(5):391–400. [PubMed] [Google Scholar]
- KIBRICK S., MELENDEZ L., ENDERS J. F. Clinical associations of enteric viruses with particular reference to agents exhibiting properties of the ECHO group. Ann N Y Acad Sci. 1957 Apr 19;67(8):311–325. doi: 10.1111/j.1749-6632.1957.tb46055.x. [DOI] [PubMed] [Google Scholar]
- MUKAI N. An evaluation of gliomas derived from the ependymal anlage. J Neuropathol Exp Neurol. 1956 Jan;15(1):33–60. doi: 10.1097/00005072-195601000-00004. [DOI] [PubMed] [Google Scholar]
- Mukai N., Kobayashi S. Extraocular orbital tumors induced by human adenovirus type 12 in hamsters. Invest Ophthalmol. 1973 Mar;12(3):185–192. [PubMed] [Google Scholar]
- Mukai N., Kobayashi S. Undifferentiated intraperitoneal tumors induced by human adenovirus type 12 in hamsters. Am J Pathol. 1972 Nov;69(2):331–348. [PMC free article] [PubMed] [Google Scholar]
- OLSON L. C., MILLER G., HANSHAW J. B. ACUTE INFECTIOUS LYMPHOCYTOSIS PRESENTING AS A PERTUSSIS-LIKE ILLNESS: ITS ASSOCIATION WITH ADENOVIRUS TYPE 12. Lancet. 1964 Jan 25;1(7326):200–201. doi: 10.1016/s0140-6736(64)92291-3. [DOI] [PubMed] [Google Scholar]
- PEREIRA M. S., MACCALLUM F. O. INFECTION WITH ADENOVIRUS TYPE 12. Lancet. 1964 Jan 25;1(7326):198–199. doi: 10.1016/s0140-6736(64)92290-1. [DOI] [PubMed] [Google Scholar]
- Pannese E. Structures possibly related to the formation of new mitochondria in spinal ganglion neuroblasts. J Ultrastruct Res. 1966 Apr;15(1):57–65. doi: 10.1016/s0022-5320(66)80093-x. [DOI] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- Popoff N., Ellsworth R. M. The fine structure of nuclear alterations in retinoblastoma and in the developing human retina: in vivo and in vitro observations. J Ultrastruct Res. 1969 Dec;29(5):535–549. doi: 10.1016/s0022-5320(69)90072-0. [DOI] [PubMed] [Google Scholar]
- SOTELO J. R., TRUJILLO-CENOZ O. Electron microscope study on the development of ciliary components of the neural epithelium of the chick embryo. Z Zellforsch Mikrosk Anat. 1958;49(1):1–12. doi: 10.1007/BF00335059. [DOI] [PubMed] [Google Scholar]
- TRENTIN J. J., YABE Y., TAYLOR G. The quest for human cancer viruses. Science. 1962 Sep 14;137(3533):835–841. doi: 10.1126/science.137.3533.835. [DOI] [PubMed] [Google Scholar]
- Tani E., Ametani T. Ciliated human astrocytoma cells. Acta Neuropathol. 1970;15(3):208–219. doi: 10.1007/BF00686767. [DOI] [PubMed] [Google Scholar]
- Tani E., Higashi N. Myelin figures associated with the cytoplasmic membranes in human medulloblastoma. Acta Neuropathol. 1972;21(2):128–139. doi: 10.1007/BF00687567. [DOI] [PubMed] [Google Scholar]
- Ts'o M. O., Fine B. S., Zimmerman L. E. The nature of retinoblastoma. II. Photoreceptor differentiation: an electron microscopic study. Am J Ophthalmol. 1970 Mar;69(3):350–359. doi: 10.1016/0002-9394(70)92264-6. [DOI] [PubMed] [Google Scholar]
- YABE Y. ANTIBODY TO ADENOVIRUS TYPE 12 AMONG INFANTS. Lancet. 1964 Jun 27;2(7348):1447–1448. doi: 10.1016/s0140-6736(64)92018-5. [DOI] [PubMed] [Google Scholar]