Abstract
The pathogenesis of acute meningoencephalitis induced in adult mice by intravenous inoculation with Semliki Forest virus has been assessed by counting cells in cerebrospinal fluid (CSF). Meningitis was first apparent on day 4 and, by the time that animals were moribund 2 days later, each microliter of CSF contained in excess of 10,000 mononuclear cells. The following conclusions were made concerning this very considerable inflammatory response: a) Complete suppression of cellular infiltration makes no difference to the clinical disease. b) No correlation is apparent between inflammation and levels of circulating antibody. c) Participation of thymus-derived lymphocytes (T cells) is essential for full expression, though not for initiation, of cellular invasion. d) There is evidently no requirement for lymphocytes recently derived from thymus or for any humoral factor secreted by thymus tissue. e) T cells entering the recirculating pool more than 6 weeks or less than about 1 week prior to inoculation of virus are equally effective in promoting inflammation. f) The T cells apparently act directly by enhancing infiltration of other blood-borne mononuclears into the brain and CSF.
Full text
PDF


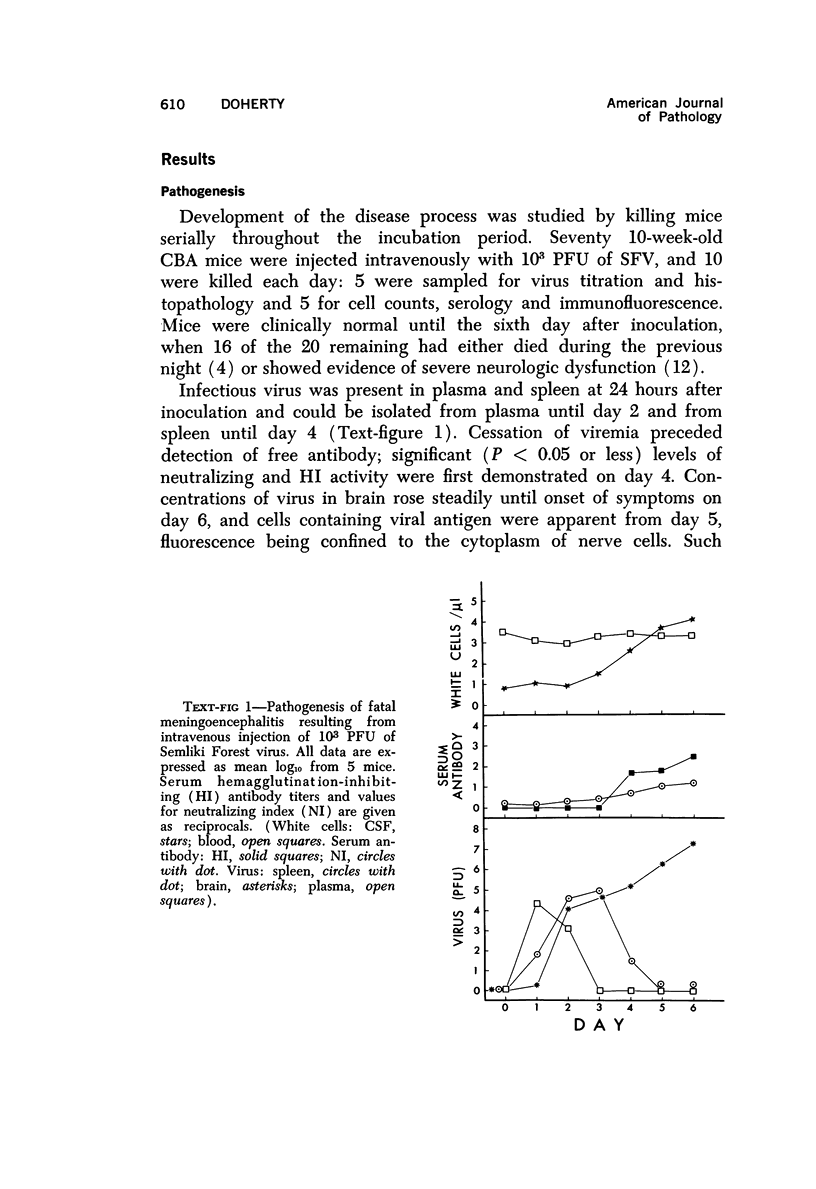
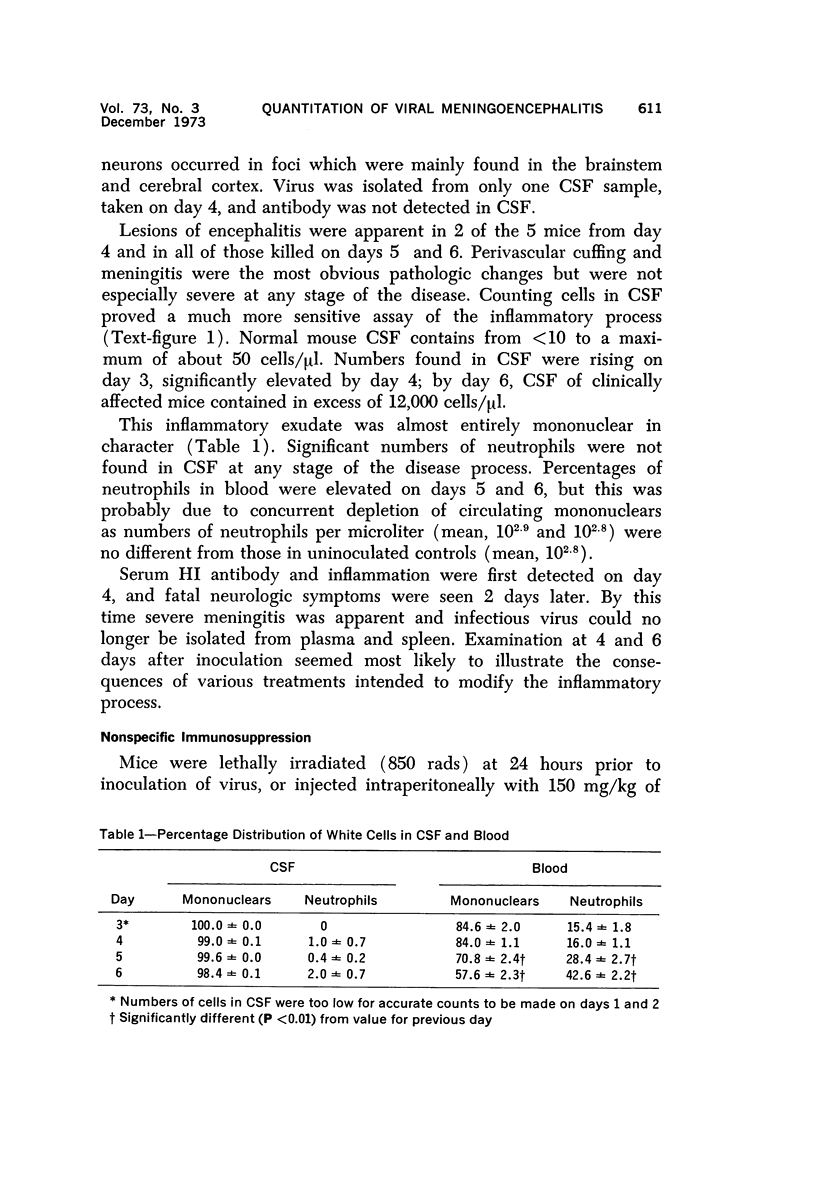
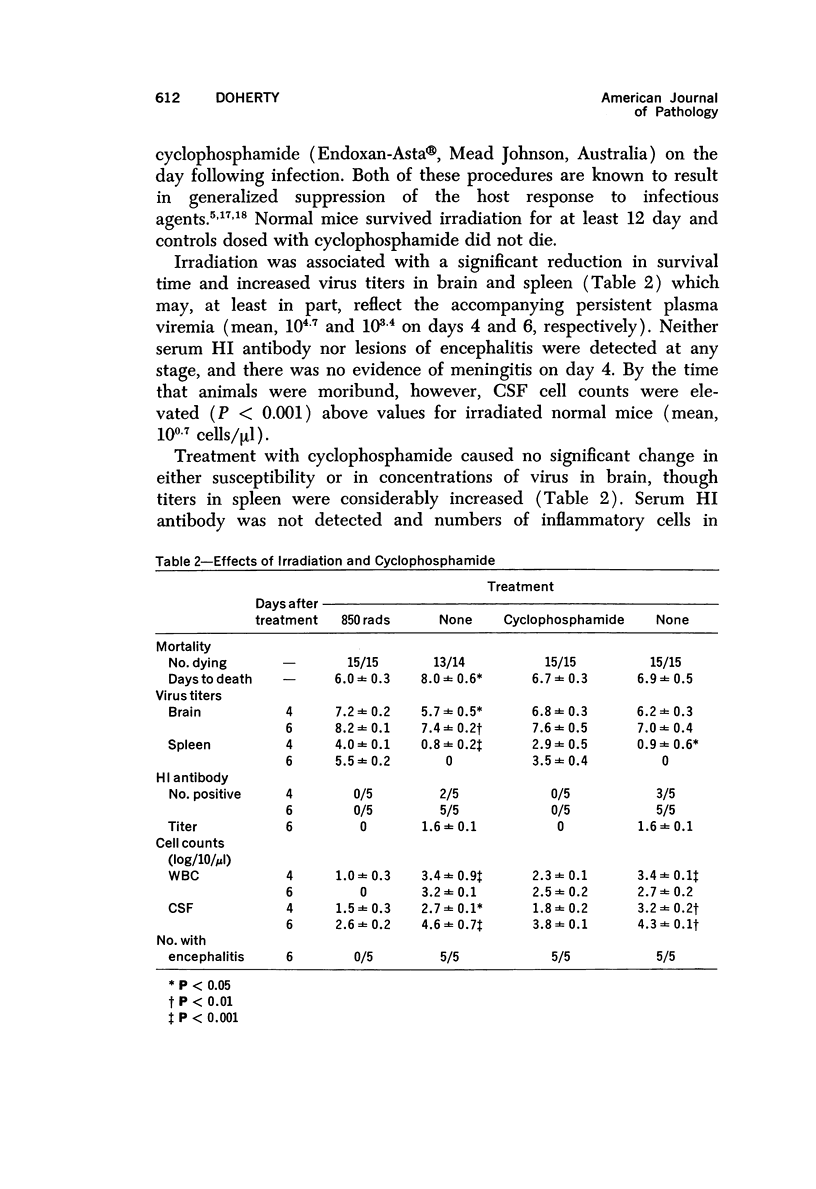

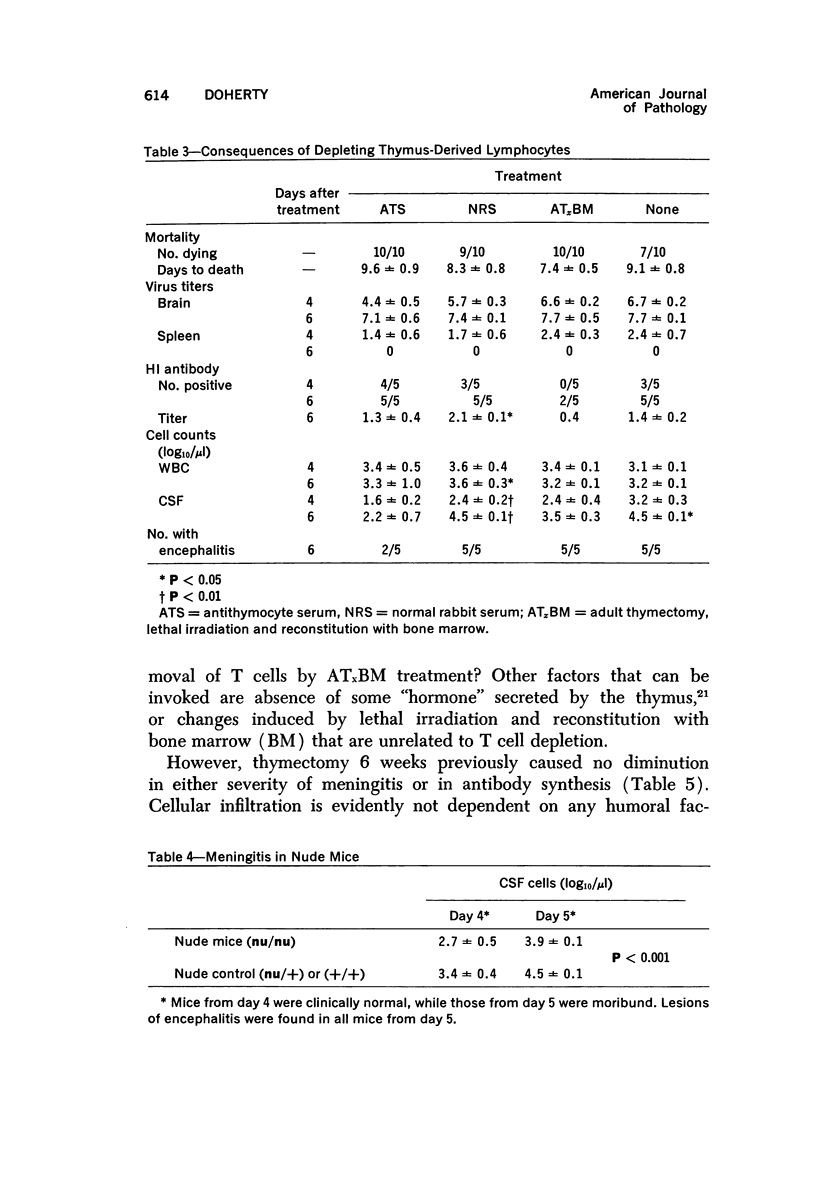





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billingham M. E., Robinson B. V., Gaugas J. M. 2 anti-inflammatory components in antilymphocytic serum. Nature. 1970 Jul 18;227(5255):276–277. doi: 10.1038/227276a0. [DOI] [PubMed] [Google Scholar]
- Blanden R. V., Langman R. E. Cell-mediated immunity to bacterial infection in the mouse. Thymus-derived cells as effectors of acquired resistance to Listeria monocytogenes. Scand J Immunol. 1972;1(4):379–391. doi: 10.1111/j.1365-3083.1972.tb03304.x. [DOI] [PubMed] [Google Scholar]
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. 3. Regression infectious foci. J Exp Med. 1971 May 1;133(5):1090–1104. doi: 10.1084/jem.133.5.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. I. The effects of anti-thymocyte serum. J Exp Med. 1970 Nov;132(5):1035–1054. doi: 10.1084/jem.132.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. II. Passive transfer of recovery mechanisms with immune lymphoid cells. J Exp Med. 1971 May 1;133(5):1074–1089. doi: 10.1084/jem.133.5.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier A. M., Snyderman R., Mergenhagen S. E., Notkins A. L. Inflammation and herpes simplex virus: release of a chemotaxis-generating factor from infected cells. Science. 1970 Dec 4;170(3962):1104–1106. doi: 10.1126/science.170.3962.1104. [DOI] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- CROSS A. M., LEUCHARS E., MILLER J. F. STUDIES ON THE RECOVERY OF THE IMMUNE RESPONSE IN IRRADIATED MICE THYMECTOMIZED IN ADULT LIFE. J Exp Med. 1964 May 1;119:837–850. doi: 10.1084/jem.119.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp R. I., Davidson A. L., Merz P. A. A method for obtaining cerebrospinal fluid from mice. Res Vet Sci. 1971 Sep;12(5):499–499. [PubMed] [Google Scholar]
- Cheville N. F. The influence of thymic and bursal lymphoid systems in the pathogenesis of avian encephalomyelitis. Am J Pathol. 1970 Jan;58(1):105–125. [PMC free article] [PubMed] [Google Scholar]
- Cole G. A., Nathanson N., Prendergast R. A. Requirement for theta-bearing cells in lymphocytic choriomeningitis virus-induced central nervous system disease. Nature. 1972 Aug 11;238(5363):335–337. doi: 10.1038/238335a0. [DOI] [PubMed] [Google Scholar]
- Davies A. J., Leuchars E., Wallis V., Doenhoff M. J. A system for lymphocytes in the mouse. Proc R Soc Lond B Biol Sci. 1971 Jan 12;176(1045):369–384. doi: 10.1098/rspb.1971.0001. [DOI] [PubMed] [Google Scholar]
- Denman A. M., Frenkel E. P. Mode of action of anti-lymphocyte globulin. I. The distribution of rabbit anti-lymphocyte globulin injected into rats and mice. Immunology. 1968 Jan;14(1):107–113. [PMC free article] [PubMed] [Google Scholar]
- Doenhoff M. J., Davies A. J., Leuchars E., Wallis V. The thymus and circulating lymphocytes of mice. Proc R Soc Lond B Biol Sci. 1970 Oct 13;176(1042):69–85. doi: 10.1098/rspb.1970.0035. [DOI] [PubMed] [Google Scholar]
- Doenhoff M. J., Davies A. J. Reconstitution of the T-cell pool after irradiation of mice. Cell Immunol. 1971 Feb;2(1):82–90. doi: 10.1016/0008-8749(71)90027-x. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Reid H. W., Smith W. Louping-ill encephalomyelitis in the sheep. IV. Nature of the perivascular inflammatory reaction. J Comp Pathol. 1971 Oct;81(4):545–549. doi: 10.1016/0021-9975(71)90083-1. [DOI] [PubMed] [Google Scholar]
- Dumonde D. C. 'Lymphokines': molecular mediators of cellular immune responses in animals and man. Proc R Soc Med. 1970 Sep;63(9):899–902. doi: 10.1177/003591577006300917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Wagner H., Basten A., Holmes M. Humoral and cell mediated responses in vitro of spleen cells from mice with thymic aplasia (nude mice). Aust J Exp Biol Med Sci. 1972 Oct;50(5):651–660. doi: 10.1038/icb.1972.57. [DOI] [PubMed] [Google Scholar]
- Gilden D. H., Cole G. A., Monjan A. A., Nathanson N. Immunopathogenesis of acute central nervous system disease produced by lymphocytic choriomeningitis virus. I. Cyclophosphamide-mediated induction by the virus-carrier state in adult mice. J Exp Med. 1972 Apr 1;135(4):860–873. doi: 10.1084/jem.135.4.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey H. M., Colón S., Campbell P., Rabellino E. Immunoglobulins on the surface of lymphocytes. V. Quantitative studies on the question of whether immunoglobulins are associated with T cells in the mouse. J Immunol. 1972 Oct;109(4):776–783. [PubMed] [Google Scholar]
- Hirsch M. S. Effects of antilymphocytic serum on host responses to infectious agents. Fed Proc. 1970 Jan-Feb;29(1):169–170. [PubMed] [Google Scholar]
- Jeejeebhoy H. F., Singla O. Mode of recovery from the effects of heterologous anti-lymphocyte serum. I. Recovery of the immune response. Immunology. 1972 May;22(5):789–799. [PMC free article] [PubMed] [Google Scholar]
- Johnson R. T. Inflammatory response to viral infection. Res Publ Assoc Res Nerv Ment Dis. 1971;49:305–312. [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey R. H. Influences of antilymphocyte serum on cell-mediated and antibody-mediated responses. Fed Proc. 1970 Jan-Feb;29(1):156–158. [PubMed] [Google Scholar]
- McFarland H. F., Griffin D. E., Johnson R. T. Specificity of the inflammatory response in viral encephalitis. I. Adoptive immunization of immunosuppressed mice infected with Sindbis virus. J Exp Med. 1972 Aug 1;136(2):216–226. doi: 10.1084/jem.136.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F. The thymus and the immune system. Vox Sang. 1971 Jun;20(6):481–491. doi: 10.1111/j.1423-0410.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Mori R., Kimoto K., Takeya K. The role of the thymus in antibody production and in resistance to Japanese encephalitis virus infection. Arch Gesamte Virusforsch. 1970;29(1):32–38. doi: 10.1007/BF01253877. [DOI] [PubMed] [Google Scholar]
- Nathanson N., Cole G. A. Immunosuppression: a means to assess the role of the immune response in acute virus infections. Fed Proc. 1971 Nov-Dec;30(6):1822–1830. [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. I. Relationship of antibody production to disease in neonatally infected mice. J Exp Med. 1969 Mar 1;129(3):483–505. doi: 10.1084/jem.129.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. II. Relationship of the anti-lymphocytic choriomeningitis immune response to tissue injury in chronic lymphocytic choriomeningitis disease. J Exp Med. 1970 Jan 1;131(1):1–19. doi: 10.1084/jem.131.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Wortis H. H. Thymus dependence of theta-bearing cells in the peripheral lymphoid tissues of mice. Immunology. 1970 Jun;18(6):931–942. [PMC free article] [PubMed] [Google Scholar]
- Ridley A. The effect of cyclophosphamide and antilymphocytic serum on the cellular reactions to homologous skin implanted into brain. Acta Neuropathol. 1970;15(4):351–358. doi: 10.1007/BF00684732. [DOI] [PubMed] [Google Scholar]
- Roessmann U., Friede R. L. Entry of labeled monocytic cells into the central nervous system. Acta Neuropathol. 1968 Jun 7;10(4):359–362. doi: 10.1007/BF00690711. [DOI] [PubMed] [Google Scholar]
- Rothwell T. L., Spector W. G. The effect of neonatal and adult thymectomy on the inflammatory response. J Pathol. 1972 Sep;108(1):15–21. doi: 10.1002/path.1711080103. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Wohlenberg C., Notkins A. L. Inflammation and viral infection: chemotactic activity resulting from the interaction of antiviral antibody and complement with cells infected with herpes simplex virus. J Infect Dis. 1972 Aug;126(2):207–209. doi: 10.1093/infdis/126.2.207. [DOI] [PubMed] [Google Scholar]
- Stockman G. D., Heim L. R., South M. A., Trentin J. J. Differential effects of cyclophosphamide on the B and T cell compartments of adult mice. J Immunol. 1973 Jan;110(1):277–282. [PubMed] [Google Scholar]
- Turk J. L., Poulter L. W. Selective depletion of lymphoid tissue by cyclophosphamide. Clin Exp Immunol. 1972 Feb;10(2):285–296. [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Cohen S., Flanagan T. D. Leukotactic factors elaborated by virus-infected tissues. J Exp Med. 1972 May 1;135(5):1095–1103. doi: 10.1084/jem.135.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Remold H. G., David J. R. Leukotactic factor produced by sensitized lymphocytes. Science. 1969 Mar 7;163(3871):1079–1081. doi: 10.1126/science.163.3871.1079. [DOI] [PubMed] [Google Scholar]
- Webb H. E., Wight D. G., Wiernik G., Platt G. S., Smith C. E. Langat virus encephalitis in mice. II. The effect of irradiation. J Hyg (Lond) 1968 Sep;66(3):355–364. doi: 10.1017/s002217240004122x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher M. C., Schmuñis G. A., Parodi A. S. Junin virus multiplication in thymectomized mice. Effect of thymus and immunocompetent cells grafting. Arch Gesamte Virusforsch. 1969;26(1):63–73. doi: 10.1007/BF01241176. [DOI] [PubMed] [Google Scholar]
- Woodbury D. M. Distribution of nonelectrolytes and electrolytes in the brain as affected by alterations in cerebrospinal fluid secretion. Prog Brain Res. 1968;29:297–314. doi: 10.1016/S0079-6123(08)64164-3. [DOI] [PubMed] [Google Scholar]



